急性肺损伤(acute lung injury, ALI)/急性呼吸窘迫综合征(acute respiratory distress syndrome, ARDS)是ICU中常见且危重的疾病,病死率约30%~45%[1]。肺内、肺外的多种高危因素均可导致其发病[2],尽管病因不同,但共同的病理生理特征是难治性低氧血症和进行性呼吸窘迫[3],其发病机制目前仍未阐明。治疗也因原发病各异而变得复杂,疗效有限,目前临床中主要采用小潮气量肺保护性通气策略、液体管理以及CRRT、ECMO等昂贵设备的应用[4-7]。因此急需寻找新的治疗方法来改善现状,脂肪干细胞(adipose-derived stem cells, ADSCs)是近年来新发现的一种充质干细胞,具有取材方便、免疫反应低等优点引起研究的关注。本研究用脂多糖(lipopolysaccharide, LPS)诱导大鼠ALI模型,探讨ADSCs在肺部的定值情况及治疗情况。
1 材料与方法 1.1 ADSCs细胞的培育及标记购买广州赛业生物科技有限公司第2代ADSCs,批号:150921131,分化到第4代时加入含25 μmol/L的EdU细胞培养液继续培育24 h备用。
1.2 实验动物分组及ALI模型制备健康雄性SD大鼠30只,体质量约300 g,由郑州大学实验动物中心提供,动物合格证号:41003100013187。按随机数字表法将大鼠分为对照组(10只)、脂多糖(LPS)模型组(10只)、LPS+ADSCs组(10只)。对照组腹腔注射生理盐水4 mL/kg,LPS组及LPS+ADSCs组腹腔注射LPS(美国Sigma公司,配制成2 mg/mL溶液)8 mg/kg制备ALI动物模型;对照组及LPS组30 min后尾静脉注射300 μL生理盐水,LPS+ADSCs组于制模30 min后尾静脉注射300 μL ADSCs(约1×106个进行干预。本实验动物处置方法符合动物伦理学标准。
1.3 检测指标及方法于实验24 h后开胸取大鼠心脏血标本及肺组织备检。
1.3.1 肺组织病理学观察取左肺上叶固定于多聚甲醛溶液中,常规石蜡包埋、切片,苏木精-伊红(HE)染色后光镜(日本Olympus公司,型号:U-CTR30-2)下观察肺损伤程度。
1.3.2 肺组织荧光染色切片进行Apollo染色及DNA染色后在荧光显微镜(德国Leica公司, 型号:DMI6000B)下观察ADSCs在肺部的定植分部情况。
1.3.3 肺湿/干质量(W/D)比值取左肺上叶,称湿质量后烤箱烤干48 h称干质量,计算肺W/D比值。
1.3.4 肿瘤坏死因子α(TNF-α)和白细胞介素-4(IL-4)水平测定取左心室血5 mL,离心取血清,用酶联免疫吸附试验(ELISA)检测血清TNF-α、IL-4含量,按试剂盒(武汉华美生物工程有限公司)说明书操作。
1.4 统计学方法用SPSS 21.0软件分析数据,计量资料以均数±标准差(x±s)表示。生存分析采用Kaplan-Meier法进行,对各组数据进行正态检验和方差齐性分析,组间两两比较用LSD-t检验,以P < 0.05为差异有统计学意义。
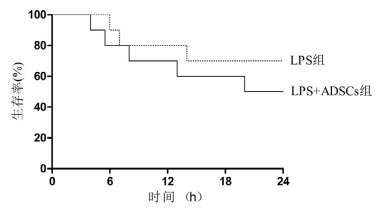
2 结果 2.1 24 h生存情况与LPS组比较,LPS+ADSCs组大鼠在24 h生存时间差异无统计学意义(50% vs. 70%, P > 0.05),见图 1。
|
| 图 1 两组生存时间曲线 Figure 1 Survival time of the two groups |
|
|
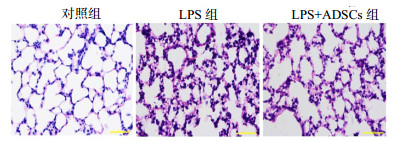
对照组肺组织结构清晰无损伤,肺泡结构完整,肺泡腔内无水肿及炎症细胞渗出改变;LPS组肺组织结构损伤较重,肺间质增厚,大量炎症细胞浸润,肺泡腔红细胞漏出,弥漫性充血水肿、渗出,严重者可见大片肺泡萎陷不张及肺泡断裂,提示模型复制成功;LPS+ADSCs组肺组织出血、渗出、肺泡萎陷及间质增宽等结构改变均较LPS模型组减轻,见图 2。
|
| 图 2 鼠肺组织病理学改变(HE×400) Figure 2 Pathological changes of lung tissue in rats (HE×400) |
|
|
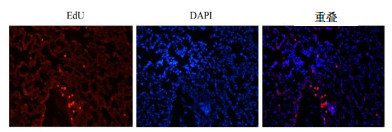
EdU染色ADSCs后的阳性细胞表现:Apollo染色ADCSs细胞核呈红色,细胞质不着色。肺组织用Hoechst33342染背景细胞,蓝色荧光位于细胞核,重叠后可见标记的ADSCs定植情况。可见用EdU标记的ADSCs通过大鼠尾静脉注入后24 h均有不同程度的在肺部定植,见图 3。
|
| 图 3 EdU标记ADSCs后的肺组织定植(×100) Figure 3 The colonization of EdU labeling ADSCs in lung tissue(×100) |
|
|
LPS组及LPS+ADSCs组肺W/D比值均明显高于正常对照组(均P < 0.01)。LPS+ADSCs组肺W/D比值较LPS组下降(P < 0.05),见表 1。
| 组别 | W/D | TNF-α(ng/L) | IL-4(pg/mL) |
| 对照组 | 4.99±0.17 | 6.07±1.24 | 10.93±3.84 |
| LPS组 | 5.98±0.28a | 45.52±3.74a | 3.27±0.54a |
| LPS+ADSCs组 | 5.57±0.27ab | 41.51±4.14ac | 7.01±1.11ac |
| F值 | 33.02 | 414.90 | 13.75 |
| 注:与对照组比较,aP < 0.01;与LPS组比较,bP < 0.01,cP < 0.05 | |||
与对照组比较,LPS组肺组织损伤较重,肺W/D比值、血TNF-α水平均显著增加(均P < 0.01),IL-4水平显著降低(P < 0.01)。与LPS组比较,LPS+ADSCs组肺组织损伤程度明显减轻,血TNF-α水平明显降低(P < 0.05),IL-4水平升高(P < 0.05),见表 1。
3 讨论具有高危因素如肺炎、禽流感、脓毒症、重症胰腺炎等肺内肺外严重疾病的患者有7%~34%进展为ALI/ARDS[8]。其发病率高、病死率高,缺乏特异的生物标志物、治疗困难以及高昂的医疗成本等问题一直困扰着医疗界[9],成为全球性重要的公共卫生问题,尽管在过去的二十年里新治疗方法及重症监护病房的建立已有显著的改善。目前动物研究常用LPS腹腔注射的方法建立ALI模型[10]。
以往对充质干细胞(MSCs)的研究主要集中在骨髓间充质干细胞(BMSCs),多项研究证明MSCs在心肌梗死、糖尿病、急性肾衰竭、结肠炎等多种疾病中取得较好的疗效[11-13]。同时研究发现在脓毒症中具有免疫调节、抗氧化、抗细胞凋亡等功能[14]。然而BMSCs增殖分化能力随年龄的增长而下降,细胞的获取需要骨髓有创穿刺。这些自身的缺陷,在今后的临床研究中可能会被限制。ADSCs是从脂肪组织中分离出来,目前越来越多的证据支持,脂肪组织更适合间充质干细胞的来源[15]。部分研究发现ADSCs能分泌更高水平的生物活性物质,较BMSCs具有更大的抗炎性潜能[16]。迄今为止,关于ADSCs对脓毒症及肺损伤模型疗效的报道很少。本研究发现大鼠腹腔注射LPS后能显著增加炎症因子TNF-α水平,而抗炎因子IL-4成严重抑制状态。黄昭等[17]研究发现TNF-α水平与ARDS肺损伤呈正相关,与生存率呈负相关。而IL-4的水平与ARDS患者预后呈正相关[18]。失控的炎症反应和抗炎反应的平衡失调是导致ADSC发生发展的关键,因此抑制过度的炎症反应、提高抗炎因子水平成治疗ALI/ARDS的切入点。本研究发现ADSCs能减轻LPS诱导ALI大鼠肺部炎症反应,减轻肺水肿,减轻血TNF-α水平,在机体免疫麻痹状态下,ADSCs能提高抗炎因子IL-4水平。
在动物模型中,最常用的移植方法是直接注射到损伤部位,这样能保证足够数量的干细胞到达目标器官,但这种方法可能因侵入性治疗导致进一步损害,因此在人体实验中变得困难。通过周围静脉注射是理想而微创的方法,然而静脉移植细胞的作用及细胞的分布是争议的问题。至于干细胞在ALI的作用机制一直饱受研究者们的争议,一部分研究者认为静脉注射MSCs能通过血液循环截留于肺组织或归巢于受损部位,抑制炎症反应,并在微环境中分化为上皮细胞、内皮细胞而起修复作用[19]。而另一部分研究者认为干细胞不能在受损的肺组织定植或定植数量十分有限,干细胞的治疗作用与定植修复机制无关,这可能通过旁分泌或内分泌机制间接治疗的结果[20]。然而干细胞在修复ALI中的准确机制尚不清楚。本研究发现EdU标记的ADSCs尾静脉注射入ALI动物模型后,经血液循环能定植到肺组织,说明ADSCs发挥治疗作用可能与在损伤部位的归巢相关。
本研究发现经腹腔注射LPS 8 mg/kg后从4 h起大鼠出现死亡,24 h LPS组与LPS+ADSCs组病死率差异无统计学意义,可能与样本量小有关。
本研究的不足之处:样本量有限,虽然该机制可以概述本研究参数之间的可能关系,但确切机制可能会更复杂,可能涉及多个代偿途径,需要更深入的研究澄清。
综上所述,本研究表明用EdU标记的ADSCs可定植在LPS诱导ALI大鼠的肺组织,能减轻ALI大鼠肺部炎症,减轻肺水肿,降低血清TNF-α水平,提高抗炎因子IL-4水平,发挥对肺组织的保护作用。
| [1] | Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries[J]. JAMA, 2016, 315(8): 788-800. DOI:10.1001/jama.2016.0291 |
| [2] | Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome[J]. J Clin Invest, 2012, 122(8): 2731-2740. DOI:10.1172/JCI60331 |
| [3] | Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury[J]. Cell, 2008, 133(2): 235-249. DOI:10.1016/j.cell.2008.02.043 |
| [4] | Umbrello M, Formenti P, Bolgiaghi L, et al. Current concepts of ards: a narrative review[J]. Int J Mol Sci, 2016, 18(1): pii: E64. DOI:10.3390/ijms18010064 |
| [5] | Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial[J]. Lancet, 2009, 374(9698): 1351-1363. DOI:10.1016/S0140-6736(09)61069-2 |
| [6] | Silversides JA, Major E, Ferguson AJ, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis[J]. Intensive Care Med, 2017, 43(2): 155-170. DOI:10.1007/s00134-016-4573-3 |
| [7] | 才权, 刘晓伟, 刘志. 急诊ARDS患者的早期识别与救治[J]. 中华急诊医学杂志, 2016, 25(3): 268-270. DOI:10.3760/cma.j.issn.1671-0282 |
| [8] | Maca J, Jor O, Holub M, et al. Past and present ards mortality rates: a systematic review[J]. Resp Care, 2017, 62(1): 113-122. DOI:10.4187/respcare.04716 |
| [9] | 郭彦利, 邱颖, 陈满秋. 急性呼吸窘迫综合征的生物标志物研究进展[J]. 中华急诊医学杂志, 2016, 25(4): 542-545. DOI:10.3760/cma.j.issn.1671-0282 |
| [10] | Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury[J]. Am J Physiol Lung Cell Mol Physiol, 2008, 295(3): L379-399. DOI:10.1152/ajplung.00010 |
| [11] | Gonzalez MA, Gonzalez-Rey E, Rico L, et al. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses[J]. Gastroenterology, 2009, 136(3): 978-989. DOI:10.1053/j.gastro.2008.11.041 |
| [12] | Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial[J]. Dis Colon Rectum, 2009, 52(1): 79-86. DOI:10.1007/DCR.0b013e3181973487 |
| [13] | Wallner C, Abraham S, Wagner JM, et al. Local application of isogenic adipose-derived stem cells restores bone healing capacity in a type 2 diabetes model[J]. Stem Cell Transl Med, 2016, 5(6): 836-844. DOI:10.5966/sctm.2015-0158 |
| [14] | Mei SHJ, McCarter SD, Deng YP, et al. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1[J]. Plos Med, 2007, 4(9): 1525-1537. DOI:10.1371/journal.pmed.0040269 |
| [15] | Zhu Y, Liu T, Song K, et al. Adipose-derived stem cell: a better stem cell than BMSC[J]. Cell Biochem Funct, 2008, 26(6): 664-675. DOI:10.1002/cbf.1488 |
| [16] | Yousefi F, Ebtekar M, Soudi S, et al. In vivo immunomodulatory effects of adipose-derived mesenchymal stem cells conditioned medium in experimental autoimmune encephalomyelitis[J]. Immunol Lett, 2016, 172: 94-105. DOI:10.1016/j.imlet.2016.02.016 |
| [17] | 黄昭, 陈裕胜, 杨自力, 等. 血管外肺水指标在脓毒症合并急性肺损伤/急性呼吸窘迫综合征患者中的作用[J]. 中华急诊医学杂志, 2012, 21(3): 244-248. DOI:10.3760/cma.j.issn.1671-0282.2012.03.004 |
| [18] | D'Alessio FR, Craig JM, Singer BD, et al. Enhanced resolution of experimental ARDS through IL-4-mediated lung macrophage reprogramming[J]. Am J Physiol Lung Cell Mol Physiol, 2016, 310(8): L733-746. DOI:10.1152/ajplung.00419.2015 |
| [19] | Kilroy GE, Foster SJ, Wu X, et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors[J]. J Cell Physiol, 2007, 212(3): 702-709. DOI:10.1002/jcp.21068 |
| [20] | Bentzon JF, Stenderup K, Hansen FD, et al. Tissue distribution and engraftment of human mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene[J]. Biochem Biophys Res Commun, 2005, 330(3): 633-640. DOI:10.1016/j.bbrc.2005.03.072 |
 2018, Vol. 27
2018, Vol. 27




