40% ~ 50%的脓毒症患者伴有心功能障碍,其中7%的患者可最终发展为严重的心力衰竭[1-3]。寻求一种理想的体外细胞模型是脓毒性心肌病机制研究的关键。目前,hiPS-CMs与人体心肌细胞有着相似的电生理和药理特性,已成为心脏疾病机制研究的良好选择[4]。实时无标记细胞分析技术(xCELLigence real-Time cell analyser cardio system, RTCA))能以无标记、无创和实时动态记录细胞信号,能很好展现药物诱导的心肌细胞的电生理变化和评估心肌毒性[5-6]。因此,本研究通过构建脂多糖(lipopolysaccharides,LPS)刺激hiPS-CMs和大鼠CMs,观察心肌细胞的电生理和炎性基因表达改变,以寻求一种更为适合作为人类脓毒性心肌病的细胞模型。
1 材料与方法 1.1 实验动物及细胞SD大鼠新生乳鼠(0~3 d)购于维通利华实验动物技术有限公司(北京),经动物伦理委员会许可,严格遵守《实验动物管理条例》进行所有实验操作。
1.2 乳鼠原代心肌细胞及hiPS-CMs制备培养参照文献的方法[7]培养乳鼠原代心肌细胞。hiPS-CMs购于北京赛贝生物技术有限公司公司,使用CardioEasy®人心肌细胞维持培养和分化成hiPS-CMs。
1.3 细胞活力和电生理检测xCELLigence RTCA Cardio软件实时监测不同浓度LPS对hiPS-CMs和原代新生大鼠CMs的细胞活力和电生理。hiPS-CMs和大鼠CMs接种后,RTCA Cardio检测到正常跳动(节律性跳动,振幅0.02以上)后,开始LPS(购于Sigma公司)干预处理。LPS处理前,孔内换液为100 μL心肌维持培养基,待细胞恢复正常跳动后,加入100 μL含LPS心肌维持培养基,使每孔LPS终浓度依次为4 μg/mL、2 μg/mL、500 ng/mL、125 ng/mL及阴性对照。在hiPS-CMs中,将LPS终浓度依次增加至40 μg/mL、20 μg/mL、10 μg/mL、5 μg/mL、2.5 μg/mL及阴性对照,每组5个复孔,采集加药前后共计30 h数据,数据采集频率为30 min/次。
1.4 RNA提取及逆转录和qPCR反应按照RNeasy Mini Kit(Qiagen公司)的说明书操作,采用Prime Script反转录试剂盒进行RNA逆转录,将反转录产物分装,置于-80℃保存。按照SYBR® Premix ExTaq试剂盒(Takara公司)的操作指南进行qPCR反应。
1.5 统计学方法使用xCELLigence RTCA Cardio软件和GraphPad Prism 6.0分析数据。以均数±标准差(Mean±SD)表示,采用单因素方差分析(one-way ANOVA)或成组t检验(Student 's t test)进行统计分析,以P < 0.05为差异有统计学意义。
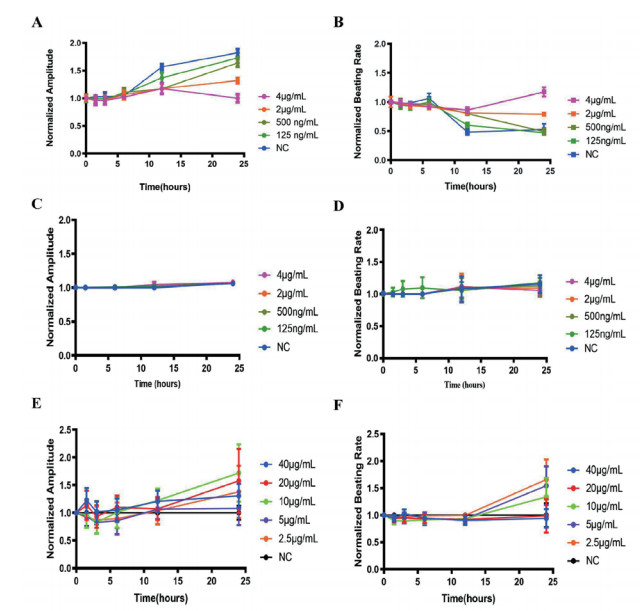
2 结果 2.1 RTCA实时监测LPS对hiPS-CMs和原代新生大鼠CMs的细胞活性和收缩情况LPS诱导48 h后,hiPS-CMs的细胞活性在LPS为5 μg/mL时出现细胞活性降低,新生大鼠CMs则在LPS为7.8 ng/mL就出现细胞活性降低。原代新生大鼠CMs的搏动频率和搏动振幅在0 h、1.5 h、3 h、6 h各浓度间差异无统计学意义,在12 h和24 h时间点则以剂量依赖的方式显著诱导收缩功能障碍,表现为搏动幅度减少和搏动频率增加(图 1A和1B)。在原代新生大鼠CMs对应的LPS浓度和时间点,hiPS-CMs搏动频率和搏动幅度均无差异性变化(图 1C和1D)。增加LPS浓度(2.5 μg/mL ~ 40 μg/mL),hiPS-CMs搏动频率和搏动幅度无明显变化(图 1E和1F)。结果表明,与hiPS-CMs比较,LPS诱导的原代新生大鼠CMs收缩功能损伤变化更敏感。
|
| 原代新生大鼠在LPS(0~4 μg/mL)作用下的搏动幅度(A)和搏动频率(B);hiPS-CMs在低浓度LPS(0~4 μg/mL)作用下的搏动幅度(C)和搏动频率(D)和hiPS-CMs在高浓度LPS(2.5~40 μg/mL)作用下的搏动幅度(E)和搏动频率(F) 图 1 LPS对hiPS-CMs和原代新生大鼠CMs的收缩作用 Fig 1 Contractile effect of LPS on hiPS-CMs and neonatal rat CMs |
|
|
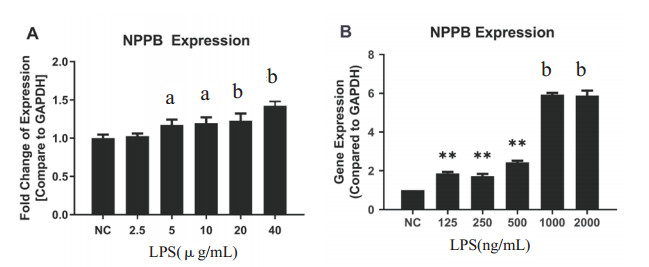
不同LPS浓度(范围为2.5 μg/mL至4 μg/mL)对hiPS-CMs的NPPB的表达没有明显改变;当LPS浓度逐步增加到40 μg/mL时,NPPB的表达水平呈剂量依赖性上升(图 2A)。在原代新生大鼠CMs中,LPS导致NPPB mRNA表达在125 ng/mL到2 μg/mL范围内就显著上调(图 2B)。这些结果表明,与hiPS-CMs相比,LPS诱导更容易导致原代新生大鼠CMs的心肌损伤。
|
| 不同LPS浓度对hiPS-CMs(A)和原代新生大鼠CMs (B)24 h NPPB mRNA的影响。与对照组比较, aP < 0.05, bP < 0.01。 图 2 LPS对hiPS-CMs和原代新生大鼠CMs的损伤影响 Fig 2 LPS-induced cell injury on hiPS-CMs and neonatal rat CMs |
|
|
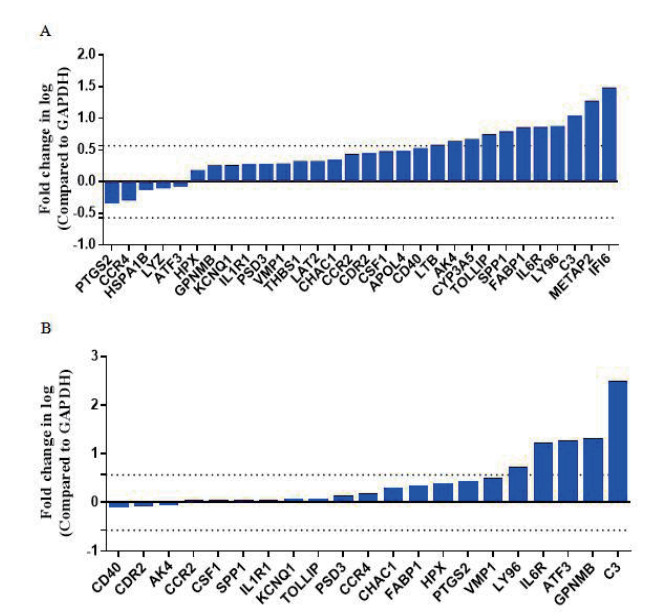
通过之前在hiPS-CMs和原代新生大鼠CMs中报道的炎症相关基因来评估LPS可能启动的炎症分子机制,如图 3所示,在LPS处理下,hiPS-CMs中的AK4、TOLLIP、SPP1、FABP1、IL6R、LY96和C3(图 3A)均上调至1.5倍;与hiPS-CMs中炎症相关基因的表达有所不同,原代新生大鼠中上调1.5倍的基因为:C3、Gpnmb、Atf3、Il6r和Ly96(图 3B)。这些结果表明,在LPS诱导下,hiPS-CMs和原代新生大鼠CMs的部分基因表达存在明显差异,而这些不同表达模式变化的潜在机制需要进一步研究。
|
| LPS对hiPS-CMs(A)和原代新生大鼠CMs(B)的炎症相关基因的表达影响 图 3 LPS改变hiPS-CMs和原代新生大鼠CMs的炎症相关基因表达 Fig 3 LPS altered inflammatory gene expression on hiPS-CMs and neonatal rat CMs. |
|
|
由于人体心脏组织存在取材困难和不容易在体外培养的局限性,大部分基础研究将人类细胞系如HeLa和HEK293T细胞作为脓毒症心肌病机制研究的体外模型。同时,非心肌细胞缺乏产生动作电位和收缩的功能,存在电生理和心脏起源的种间差异,上述人类细胞系并不是功能研究的理想选择[8-9]。本研究通过构建LPS刺激hiPS-CMs和大鼠CMs,采用RTCA技术实时观察心肌细胞的搏动幅度和搏动频率,进一步采用qPCR阵列法检测LPS诱导脓毒性心肌病的炎症相关基因表达,以初步探讨其炎症机制。
LPS可在细胞、功能和基因组水平上诱导原代新生大鼠CMs产生心脏毒性作用,表现为NPPB基因表达上调,且呈浓度和时间依赖性,并伴有搏动频率增加,搏动幅度减小,这与以往研究一致[7]。由于离子通道、生物通路和药代动力学特性的种间差异,原代新生大鼠CMs模型不能准确预测人类心脏毒性情况[10],而hiPS-CMs具有与天然心肌细胞相似的电生理和药理特性,可作为心脏疾病建模的良好选择。本研究显示,相对应的LPS(0~4 ng/mL)处理hiPS-CMs,其收缩功能(搏动幅度和搏动频率)差异无统计学意义。并且,更高浓度的LPS(40 μg/mL),仍未能引起hiPS-CMs的搏动幅度和搏动频率发生显著改变。其次,在LPS浓度高达5 μg/mL时,NPPB基因表达水平才与对照组差异有统计学意义。这就表明,与原代新生大鼠CMs相比,hiPS-CMs对LPS更耐受。既往的研究表明,原代新生大鼠CMs和hiPS-CMs中LPS诱导的心功能障碍模式不同,这可能与靶向离子通道的化合物有关,进而影响动作电位和心脏搏动频率[11-12]。小鼠CMs的主要复极电流是瞬时外向钾电流,而不像hiPS-CMs快速延迟整流钾电流。小鼠心肌细胞比人类心肌细胞对T型Ca2+通道封锁更敏感[13]。
在LPS诱导hiPS-CMs和原代新生大鼠CMs炎症相关基因表达方面,IL6R、LY96和C3这三个基因(炎症反应的典型标记)在hiPS-CMs和原代新生大鼠CMs中均显著上调。TOLLIP1, SPP1FA、BP1和AK4仅在hiPS-CMs中上调,而ATF3和GPNMB仅在原代新生大鼠CMs中发生变化。据报道,在LPS诱导下,FABP1能抑制ERK、p38和JNK的磷酸化[14],而AK4作为氧化应激的调节剂,具有应激保护功能[15],这两种基因的过度表达可能会使hiPS-CMs表现出对LPS诱导的高耐受性。然而,GPNMB(一种与炎症和心脏重构相关的基因[12])的水平在原代新生大鼠CMs模型中升高,但在hiPS-CMs模型中没有明显变化。这一观察突出了不同实验条件下炎症相关基因表达在疾病发病机制中的不同作用[17-19]。
本研究结果对LPS诱导脓毒性心肌病的细胞模型选择有一定的参考作用。hiPS-CMs和原代新生大鼠CMs均适合作为预测LPS诱导炎症反应发生的动物模型,但在研究不同的发病机制方面各有优势。例如,为了评估LPS诱导的心肌功能障碍,原代新生大鼠CMs可能是一个理想的选择,因为在LPS处理下更加敏感;近年来,hiPS-CMs常被用于研究病毒性心肌炎症和LPS诱导炎症反应的病理生理和分子机制研究[20],因此,当研究LPS诱导的炎症反应机制时,选择hiPS-CMs而不是原代新生大鼠CMs模型试验会更加合理。
利益冲突 所有作者均声明不存在利益冲突。
| [1] | 中国医疗保健国际交流促进会急诊医学分会. 中国脓毒症早期预防与阻断急诊专家共识[J]. 中华急诊医学杂志, 2020, 29(7): 885-895. DOI:10.3760/cma.j.issn.1671-0282.2020.07.001 |
| [2] | Nabzdyk CS, Couture EJ, Shelton K, et al. Sepsis induced cardiomyopathy: Pathophysiology and use of mechanical circulatory support for refractory shock[J]. J Crit Care, 2019, 54: 228-234. DOI:10.1016/j.jcrc.2019.09.001 |
| [3] | 商会棉, 王金荣, 杨晓亚, 等. 新旧指南诊断脓毒症舒张功能不全的对比研究[J]. 中华急诊医学杂志, 2020, 29(9): 1203-1209. DOI:10.3760/cma.j.issn.1671-0282.2020.09.012 |
| [4] | Shu NK, Ihara D, Hasegawa K, et al. Applications for induced pluripotent stem cells in disease modelling and drug development for heart diseases[J]. Eur Cardiol, 2020, 15: 1-10. DOI:10.15420/ecr.2019.03 |
| [5] | Asakura K, Hayashi S, Ojima A, et al. Improvement of acquisition and analysis methods in multi-electrode array experiments with iPS cell-derived cardiomyocytes[J]. J Pharmacol Toxicol Methods, 2015, 75: 17-26. DOI:10.1016/j.vascn.2015.04.002 |
| [6] | Navarrete EG, Liang P, Lan F, et al. Screening drug-induced arrhythmia corrected using human induced pluripotent stem cell-derived cardiomyocytes and low-impedance microelectrode arrays[J]. Circulation, 2013, 128(11 Suppl 1): S3-S13. DOI:10.1161/CIRCULATIONAHA.112.000570 |
| [7] | Himmel HM. Drug-induced functional cardiotoxicity screening in stem cell-derived human and mouse cardiomyocytes: effects of reference compounds[J]. J Pharmacol Toxicol Methods, 2013, 68(1): 97-111. DOI:10.1016/j.vascn.2013.05.005 |
| [8] | Mathur A, Ma Z, Loskill P, et al. In vitro cardiac tissue models: Current status and future prospects[J]. Adv Drug Deliv Rev, 2016, 96: 203-213. DOI:10.1016/j.addr.2015.09.011 |
| [9] | Pointon A, Harmer AR, Dale IL, et al. Assessment of cardiomyocyte contraction in human-induced pluripotent stem cell-derived cardiomyocytes[J]. Toxicol Sci, 2015, 144(2): 227-237. DOI:10.1093/toxsci/kfu312 |
| [10] | Scott CW, Zhang XY, Abi-Gerges N, et al. An impedance-based cellular assay using human iPSC-derived cardiomyocytes to quantify modulators of cardiac contractility[J]. Toxicol Sci, 2014, 142(2): 331-338. DOI:10.1093/toxsci/kfu186 |
| [11] | Ma JY, Guo L, Fiene SJ, et al. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents[J]. Am J Physiol Heart Circ Physiol, 2011, 301(5): H2006-H2017. DOI:10.1152/ajpheart.00694.2011 |
| [12] | Ahmed RE, Anzai T, Chanthra N, et al. A brief review of current maturation methods for human induced pluripotent stem cells-derived cardiomyocytes[J]. Front Cell Dev Biol, 2020, 8: 178. DOI:10.3389/fcell.2020.00178 |
| [13] | Uzun AU, Mannhardt I, Breckwoldt K, et al. Ca(2+)-currents in human induced pluripotent stem cell-derived cardiomyocytes effects of two different culture conditions[J]. Front Pharmacol, 2016, 7: 300. DOI:10.3389/fphar.2016.00300 |
| [14] | Martin I, Cabán-Hernández K, Figueroa-Santiago O, et al. Fasciola hepatica fatty acid binding protein inhibits TLR4 activation and suppresses the inflammatory cytokines induced by lipopolysaccharide in vitro and in vivo[J]. J Immunol, 2015, 194(8): 3924-3936. DOI:10.4049/jimmunol.1401182 |
| [15] | Kong FZ, Binas B, Moon JH, et al. Differential expression of adenylate kinase 4 in the context of disparate stress response strategies of HEK293 and HepG2 cells[J]. Arch Biochem Biophys, 2013, 533(1/2): 11-17. DOI:10.1016/j.abb.2013.02.014 |
| [16] | Omura S, Kawai E, Sato F, et al. Bioinformatics multivariate analysis determined a set of phase-specific biomarker candidates in a novel mouse model for viral myocarditis[J]. Circ Cardiovasc Genet, 2014, 7(4): 444-454. DOI:10.1161/CIRCGENETICS.114.000505 |
| [17] | Higa A, Hoshi H, Takagi M. Differing responses of human stem cell-derived cardiomyocytes to arrhythmogenic drugs, determined using impedance measurements[J]. Fundam Toxicol Sci, 2016, 3(2): 47-53. DOI:10.2131/fts.3.47 |
| [18] | Maherali N, Ahfeldt T, Rigamonti A, et al. A high-efficiency system for the generation and study of human induced pluripotent stem cells[J]. Cell Stem Cell, 2008, 3(3): 340-345. DOI:10.1016/j.stem.2008.08.003 |
| [19] | Jung G, Fajardo G, Ribeiro AJ, et al. Time-dependent evolution of functional vs. remodeling signaling in induced pluripotent stem cell-derived cardiomyocytes and induced maturation with biomechanical stimulation[J]. FASEB J, 2016, 30(4): 1464-1479. DOI:10.1096/fj.15-280982 |
| [20] | Yücel G, Zhao ZH, El-Battrawy I, et al. Lipopolysaccharides induced inflammatory responses and electrophysiological dysfunctions in human-induced pluripotent stem cell derived cardiomyocytes[J]. Sci Rep, 2017, 7(1): 2935. DOI:10.1038/s41598-017-03147-4 |
 2021, Vol. 30
2021, Vol. 30




