脑缺血-再灌注损伤后大量炎症因子的释放明显加重脑组织损害[1];中枢神经系统内主要的免疫细胞是小胶质细胞,它是神经炎症因子的主要来源[2]。激活的小胶质细胞可通过释放炎症因子IL-1β、TNF-α等发挥细胞毒性效应[3-4],其中IL-1β能抑制神经前体细胞增殖、分化,降低IL-1β的表达能减少脑梗死面积和神经元的凋亡[5-6]。本研究拟通过缺氧培养BV-2小胶质细胞4 h然后复氧培养24 h,以模拟脑缺血-再灌注损伤时小胶质细胞微环境的改变,探讨缺氧后小胶质细胞Notch信号通路是否激活,及其对IL-1β表达的影响,为抑制脑缺血-再灌注损伤后炎症因子的释放提供新的治疗靶点。
1 材料与方法 1.1 试剂与仪器免疫荧光显微镜(Olympus DP73 Microscope,Olympus,日本);蛋白成像仪(ImageQuant LAS 500,瑞典);逆转录试剂盒(TAKARA,大连,中国,货号:DRR036S);PCR定量试剂盒(TAKARA,大连,中国,货号:DRR820A);DAPT (Sigma,密苏里州,美国,货号:D5942);Lectin (Sigma,密苏里州,美国,货号:L0401);一抗:NICD (Cell Signaling Technology,马萨诸塞州,美国,货号:4147)、IL-1β (Abcam,马萨诸塞州,美国,货号:Ab9787);二抗:羊抗兔-HRP (Cell Signaling Technology,马萨诸塞州,美国,货号:7074)、羊抗兔荧光二抗(Life Technologies,加利福尼亚州,美国,货号:A-21428)。
1.2 细胞培养及分组BV-2小胶质细胞受赠于新加坡国立大学,先将冻存的细胞从液氮取出后迅速投入预热的水浴箱中,液体完全溶解后离心并留取细胞沉渣,用10% FBS-DMEM培养基将细胞吹打成悬液并种至75 cm2培养瓶中进行细胞培养。将培养的BV-2小胶质细胞分为对照组、缺氧无糖培养组(oxygen glucose deprivation,OGD组)、及缺氧无糖培养+ 10 µmol/L DAPT组(DAPT组);对照组细胞用10% FBS-DMEM培养基正常培养,OGD组、DAPT组缺氧无糖培养4 h(含3% O2, 5% CO2, 92%N2培养箱中培养)后更换为10% FBS-DMEM培养基在正常培养箱中复氧24 h。
1.3 CCK8检测10µmol/L DAPT对BV-2小胶质细胞的影响根据文献[7]采用10 µmol/L DAPT抑制Notch信号通路,先用CCK8分析10 µmol/L DAPT对BV-2小胶质细胞活性的影响。将细胞接种至96孔板中,培养30 min后倒去上清液以清除不贴璧细胞,加入10% FBS-DMEM培养基继续培养24 h,将细胞分为缺氧无糖培养组(OGD组)及缺氧无糖+10 µmol/L DAPT组(DAPT组),将两组细胞缺氧培养4 h再复氧24 h,每孔加入10 µL CCK8溶液,然后置于37℃恒温环境孵育4 h;再用酶标仪测定在450 nm处的吸光度,根据吸光度计算细胞活性。
1.4 Western Blot检测BV-2小胶质细胞NICD、IL-1β蛋白表达BV-2小胶质细胞分为对照组、缺氧无糖培养组(OGD组)、缺氧无糖培养+10 µ mol/L DAPT组(DAPT组),各组相应处理后提取细胞的总蛋白,然后使用10% SDS-PAGE凝胶进行电泳,再电转至PVDF膜。转膜成功后用5%的脱脂奶粉室温封闭2 h,随后孵育一抗NICD (1:300)、IL-1β (1:200)于4℃冰箱过夜。次日取出,用TBST漂洗后再4℃孵育二抗2 h。最后使用蛋白成像仪检测目的蛋白条带,使用Fluorchem 8900灰度分析软件进行灰度值分析。
1.5 RT-PCR检测BV-2小胶质细胞IL-1β mRNA的表达BV-2小胶质细胞分为对照组、缺氧无糖培养组(OGD组)、缺氧无糖培养+10 µmol/L DAPT组(DAPT组),各组相应处理后用Trizol提取总RNA并按照逆转录试剂盒说明书将提取的RNA逆转录成cDNA;按照10 µL Real Time PCR反应体系进行扩增,IL-1β扩增引物如下:F: GCCCATCCTCTGTGACTCAT,R:AGGCCACAGGTATTTTGTCG。扩增结束后用Delta-delta Ct[8]法计算各组IL-1β mRNA的表达。
1.6 免疫荧光法检测BV-2小胶质细胞IL-1β的表达BV-2小胶质细胞分为对照组、缺氧无糖培养组(OGD组)、缺氧无糖培养+10 µ mol/L DAPT组(DAPT组),各组相应处理后将玻片进行漂洗、多聚甲醛固定及BSA封闭后,加入一抗IL-1β (1:50)于4℃冰箱孵育过夜,次日室温孵育二抗(1:1 000)和Lectin (1:100) 2 h;再用5 µg/mL DAPI染核;最后加抗淬灭剂封片及随机取5个视野于免疫荧光显微镜下阅片。
1.7 统计学方法所有数据使用统计学软件SPSS 13.0进行分析,计量资料以均数±标准差(Mean±SD)表示,多组间分析时采用one-way ANOVA方差分析,两两比较分析时采用LSD-t检验方法分析,以P < 0.05为差异有统计学意义。
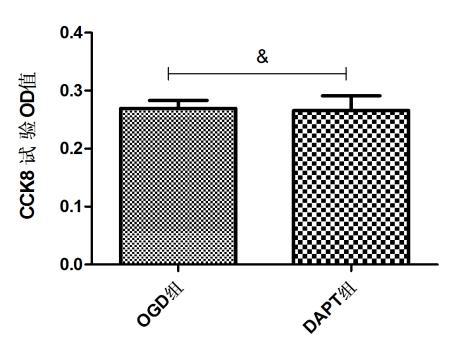
2 结果 2.1 10µmol/L DAPT对BV-2小胶质细胞活性无影响CCK8检测OGD组及DAPT组BV-2小胶质细胞的OD值分别为:0.269±0.013、0.265±0.025;经统计分析,两组细胞的OD值(P > 0.05),表明10 µ mol/L DAPT对BV-2小胶质细胞活性无影响(图 1)。
|
| 图 1 10 µmol/L DAPT对BV-2小胶质细胞活性影响 Fig 1 The effect of 10 µmol/L DAPT on the cell viability of BV-2 microglia |
|
|
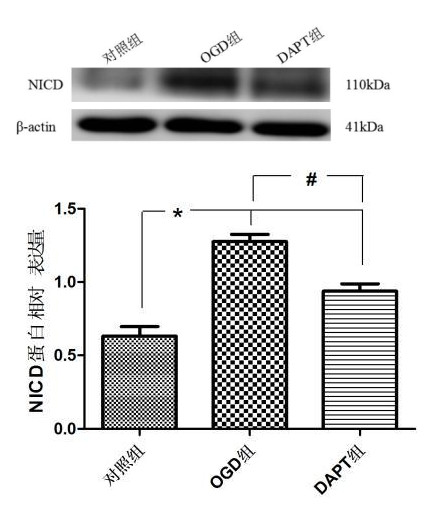
各组BV-2小胶质细胞中NICD蛋白表达量如下:对照组(0.632±0.065),OGD组(1.276±0.049),DAPT(0.938±0.049)。经统计分析(图 2):与对照比较,OGD组与DAPT组小胶质细胞NICD蛋白表达明显增加(P < 0.05);与OGD组比较,DAPT组BV-2小胶质细胞NICD蛋白表达明显减少(P < 0.05)。表明缺氧BV-2小胶质细胞Notch信号通路被激活,DAPT可抑制Notch信号通路的激活。
|
| aP<0.05,bP<0.05 图 2 Western blot检测各组NICD蛋白表达 Fig 2 The level of NICD protein expression measured by Western blot |
|
|
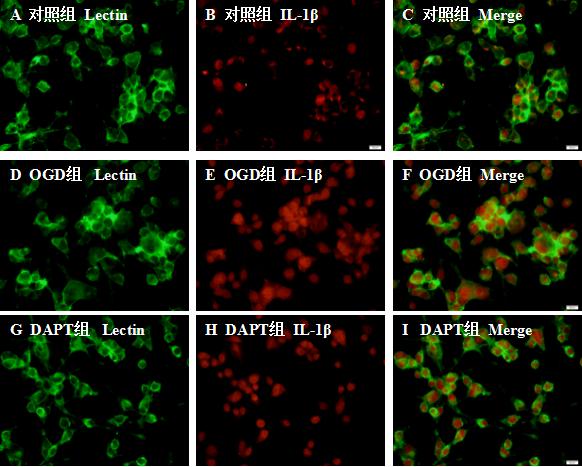
对照组BV-2小胶质细胞少量表达IL-1β;OGD组与DAPT组BV-2小胶质细胞IL-1β的表达增加;与OGD组比较,DAPT抑制Notch信号通路后BV-2小胶质细胞IL-1β的表达减少。见图 3。
|
| A、D、G代表相应各组Lectin标记的BV-2小胶质细胞,B、E、H分别表示:对照组、OGD组、DAPT组IL-1β的表达情况,C、F、I表示各组Lectin和IL-1β的共定位表达情况。水平标尺:A-I, 20 μm 图 3 免疫荧光图像显示各组Lectin和IL-1β的共定位表达 Fig 3 Co-localization expression of Lectin and IL-1β in each group by immunofl uorescence |
|
|
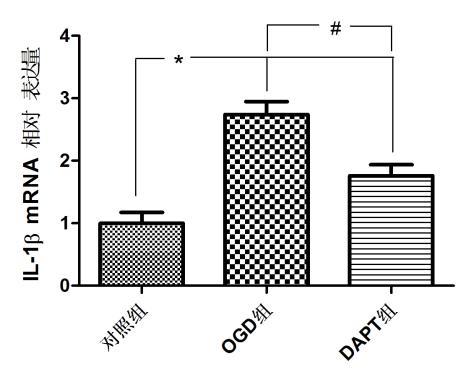
各组小胶质细胞IL-1β mRNA的相对表达量分别为:对照组(1.000±0.173),OGD组(2.741±0.207),DAPT组(1.762±0.177);:与对照组比较,OGD组与DAPT组BV-2小胶质细胞IL-1β mRNA表达明显增加(P < 0.05);与OGD组比较,DAPT组BV-2小胶质细胞IL-1β mRNA表达明显减少(P < 0.05)。表明缺氧BV-2小胶质细胞IL-1β mRNA表达增加,抑制Notch信号通路可减少缺氧BV-2小胶质细胞IL-1β mRNA表达。见图 4。
|
| aP<0.05,bP<0.05 图 4 各组IL-1β mRNA的表达水平 Fig 4 Expression level of IL-1β mRNA in each group |
|
|
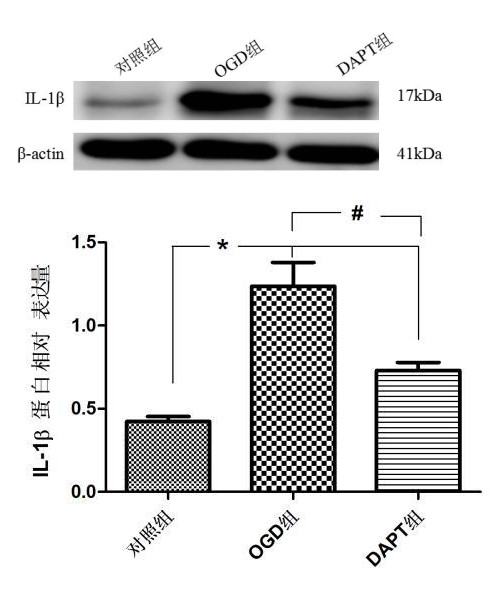
各组BV-2小胶质细胞IL-1β蛋白表达量如下:对照组(0.422±0.030),OGD组(1.236±0.143),DAPT组(0.730±0.047)。与对照比较,OGD组与DAPT组小胶质细胞IL-1β蛋白表达明显增加(P < 0.05);与OGD组比较,DAPT组BV-2小胶质细胞IL-1β蛋白表达明显减少(P < 0.05)。表明缺氧BV-2小胶质细胞IL-1β蛋白表达增加,抑制Notch信号通路可减少缺氧BV-2小胶质细胞IL-1β蛋白表达。见图 5。
|
| aP<0.05,bP<0.05 图 5 Western blot检测各组IL-1β蛋白表达 Fig 5 Western blot analysis of IL-1β protein expression in each group |
|
|
脑卒中是临床上常见的脑血管疾病,具有高发病率、致残率和病死率的特点,急性缺血性脑卒中占脑卒中的60%~80%,通过溶栓或取栓恢复前向血流是最重要的治疗措施[9]。但血流恢复后仍存在脑缺血-再灌注损伤,继续损害神经功能。其中,神经炎症因子在脑缺血-再灌注损伤中扮演重要角色,这些炎症因子主要来源于激活的小胶质细胞;小胶质细胞来源于单核巨噬细胞系统,是中枢神经系统内主要的免疫细胞,密切监视中枢神经系统微环境改变,吞噬凋亡、坏死细胞和病原微生物[2]。当缺血-再灌注损伤后,小胶质细胞率先被激活,释放大量的炎症介质及炎症因子,如iNOS,ROS,TNF-α、IL-1β,进一步促进神经炎症反应,加剧神经元的损伤和丢失[10-12]。
有临床研究表明,缺血性脑卒中患者血清中IL-1β的水平明显高于健康对照组[13-14],通过流式细胞仪检测发现缺血性脑损伤后小鼠的IL-1β主要来源于小胶质细胞和巨噬细胞[15]。IL-1β可通过对内皮细胞和胆碱能神经元的不同作用介导脑损伤[16]。此外,IL-1β可促进一系列炎症反应,这些反应可以加剧或诱导神经元损伤和死亡[17]。本研究通过缺氧培养BV-2小胶质细胞4h然后复氧培养24h,模拟脑缺血-再灌注损伤时小胶质细胞微环境的改变,发现缺氧后BV-2小胶质细胞IL-1β表达也明显增加。
目前IL-1β在脑缺血-再灌注损伤中的相关研究主要在于炎症小体NLRP3的活化,但近年来Notch信号通路在脑缺血-再灌注损伤后调控神经炎症方向备受关注。有研究表明脑缺血-再灌注损伤后Notch信号通路被激活,NICD表达明显上调,抑制Notch信号通路可明显减少神经元凋亡、降低脑梗死面积和提高神经行为功能[18]。用DAPT抑制Notch信号通路的激活后,小胶质细胞TLR4 mRNA、MyD88 mRNA、TRAF6及磷酸化NF-κB的表达明显减少[7, 19]。大量临床试验表明IL-1β是脑卒中的重要治疗靶点,与患者的预后及病死率密切相关[20],寻找与IL-1β相关的信号通路至关重要。因此,本研究探讨缺氧后小胶质细胞Notch信号通路是否激活,及其对IL-1β表达的影响。实验表明缺氧后BV-2小胶质细胞Notch信号通路被激活,IL-1β表达明显增加;通过DAPT抑制Notch信号通路后BV-2小胶质细胞IL-1β表达明显减少。
综上所述,缺氧可激活小胶质细胞Notch信号通路,而抑制Notch信号可减少缺氧小胶质细胞IL-1β的表达。本研究从新的角度探索IL-1β相关信号通路,为抑制脑缺血-再灌注损伤后神经炎症反应提供新的治疗靶点,为下一步相关临床药物研究提供新思路;但本研究仅从体外细胞水平模拟脑缺血-再灌注损伤时小胶质细胞微环境的改变,有待从动物实验水平进一步探讨抑制Notch信号通路是否减少脑缺血灶周围IL-1β的表达及提高神经行为功能评分。
利益冲突 所有作者均声明不存在利益冲突。
| [1] | Amantea D, Nappi G, Bernardi G, et al. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators[J]. FEBS J, 2009, 276(1): 13-26. DOI:10.1111/j.1742-4658.2008.06766.x |
| [2] | Abe N, Nishihara T, Yorozuya T, et al. Microglia and macrophages in the pathological central and peripheral nervous systems[J]. Cells, 2020, 9(9): E2132. DOI:10.3390/cells9092132 |
| [3] | Todd L, Palazzo I, Suarez L, et al. Reactive microglia and IL1β/IL-1R1-signaling mediate neuroprotection in excitotoxin-damaged mouse Retina[J]. J Neuroinflammation, 2019, 16(1): 118. DOI:10.1186/s12974-019-1505-5 |
| [4] | Benakis C, Garcia-Bonilla L, Iadecola C, et al. The role of microglia and myeloid immune cells in acute cerebral ischemia[J]. Front Cell Neurosci, 2014, 8: 461. DOI:10.3389/fncel.2014.00461 |
| [5] | Lu KT, Wang YW, Yang JT, et al. Effect of interleukin-1 on traumatic brain injury-induced damage to hippocampal neurons[J]. J Neurotrauma, 2005, 22(8): 885-895. DOI:10.1089/neu.2005.22.885 |
| [6] | Wang XF, Fu SL, Wang YX, et al. Interleukin-1beta mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway[J]. Mol Cell Neurosci, 2007, 36(3): 343-354. DOI:10.1016/j.mcn.2007.07.005 |
| [7] | Yao LL, Kan EM, Kaur C, et al. Notch-1 signaling regulates microglia activation via NF-κB pathway after hypoxic exposure in vivo and in vitro[J]. PLoS One, 2013, 8(11): e78439. DOI:10.1371/journal.pone.0078439 |
| [8] | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method[J]. Methods, 2001, 25(4): 402-408. DOI:10.1006/meth.2001.1262 |
| [9] | 国家卫生健康委员会急诊医学质控中心, 中国医师协会急诊医师分会, 世界中医药学会联合会急症专业委员会. 中国急性缺血性脑卒中急诊诊治专家共识[J]. 中国急救医学, 2018, 38(4): 281-287. DOI:10.3969/jz.issn.1002-1949.2018.04.001 |
| [10] | 陈燕启, 刘德红, 姜宏志, 等. 葛根素对脑缺血-再灌流时大鼠肿瘤坏死因子-α和白细胞介素-1β表达的影响[J]. 中华急诊医学杂志, 2005, 14(2): 119-121. DOI:10.3760/j.issn:1671-0282.2005.02.010 |
| [11] | Luo AL, Yan J, Tang XL, et al. Postoperative cognitive dysfunction in the aged: the collision of neuroinflammaging with perioperative neuroinflammation[J]. Inflammopharmacology, 2019, 27(1): 27-37. DOI:10.1007/s10787-018-00559-0 |
| [12] | Réus GZ, Fries GR, Stertz L, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders[J]. Neuroscience, 2015, 300: 141-154. DOI:10.1016/j.neuroscience.2015.05.018 |
| [13] | 叶心国, 李承晏, 毛善平. 急性脑梗死患者血清TNF-α、IL-1β和sICAM-1含量变化[J]. 中华急诊医学杂志, 2003, 12(1): 32-34. DOI:10.3760/j.issn:1671-0282.2003.01.011 |
| [14] | Wytrykowska A, Prosba-Mackiewicz M, Nyka WM. IL-1β, TNF-α, and IL-6 levels in gingival fluid and serum of patients with ischemic stroke[J]. J Oral Sci, 2016, 58(4): 509-513. DOI:10.2334/josnusd.16-0278 |
| [15] | Clausen BH, Lambertsen KL, Babcock AA, et al. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice[J]. J Neuroinflammation, 2008, 5: 46. DOI:10.1186/1742-2094-5-46 |
| [16] | Wong R, Lénárt N, Hill L, et al. Interleukin-1 mediates ischaemic brain injury via distinct actions on endothelial cells and cholinergic neurons[J]. Brain Behav Immun, 2019, 76: 126-138. DOI:10.1016/j.bbi.2018.11.012 |
| [17] | Tuttolomondo A, di Raimondo D, di Sciacca R, et al. Inflammatory cytokines in acute ischemic stroke[J]. Curr Pharm Des, 2008, 14(33): 3574-3589. DOI:10.2174/138161208786848739.[LinkOut |
| [18] | Arumugam TV, Chan SL, Jo DG, et al. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke[J]. Nat Med, 2006, 12(6): 621-623. DOI:10.1038/nm1403 |
| [19] | Yao LL, Cao Q, Wu CY, et al. Notch signaling in the central nervous system with special reference to its expression in microglia[J]. CNS Neurol Disord Drug Targets, 2013, 12(6): 807-814. DOI:10.2174/18715273113126660172 |
| [20] | Sobowale OA, Parry-Jones AR, Smith CJ, et al. Interleukin-1 in stroke: from bench to bedside[J]. Stroke, 2016, 47(8): 2160-2167. DOI:10.1161/STROKEAHA.115.010001 |
 2020, Vol. 29
2020, Vol. 29




