2 浙江大学医学院附属第二医院放射科
2 Department of Radiology, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310009, China
胃肠功能紊乱是危重患者常见的并发症之一,常表现为胃潴留,容易发生胃内容物反流、误吸,导致不良的临床预后[1-2]。胃残余量监测的意义重大,已被用于协助危重患者肠内营养的实施与调整[2]。传统的胃残余量监测方法主要有胃管抽吸法、磁共振、CT等,但胃管抽吸法并不能准确地反应胃残余量情况[3],磁共振和CT能比较准确地监测胃残余量[4-5],却不适于危重患者床旁实时监测的需求。床旁超声可用于胃残余量的监测,能有效地评估外科患者围手术期的误吸风险及指导术中麻醉的管理[6],但在危重患者中应用的报道并不多。本研究通过与腹部CT比较,评价床旁超声检测胃残余量的可靠性,以及预测肠内营养喂养不耐受的价值,为危重患者的胃残余量评估和指导肠内营养开展提供有效的手段。
1 资料与方法 1.1 一般资料为回顾性研究。纳入2018年4~9月浙江大学医学院附属第二医院急诊ICU内实施肠内营养的危重患者为研究对象。纳入标准:⑴年龄≥18岁;⑵体质量指数18~35 kg/m2;⑶因疾病诊治需求进行腹部CT检查者;⑷每日常规应用超声监测胃窦截面积、且在进行腹部CT检查时已完成超声监测≥48 h的患者;⑸选择腹部CT检查和胃超声监测相隔30 min内的数据。排除标准:⑴妊娠;⑵上腹部胃肠道手术史;⑶存在反流性食管炎、幽门梗阻、肠梗阻等影响胃肠动力的消化系统疾病。该研究符合医学伦理学标准,并经医院医学伦理委员会批准(批件号:2020伦审研第368号)。
1.2 研究方法回顾收集研究对象的一般资料与临床特征,包括年龄、性别、身高、体质量、体质量指数、格拉斯哥昏迷评分(glasgow coma scale, GCS)评分、急性生理和慢性健康评估Ⅱ(acute physiology and chronic health evaluation Ⅱ, APACHE Ⅱ)、疾病诊断等。
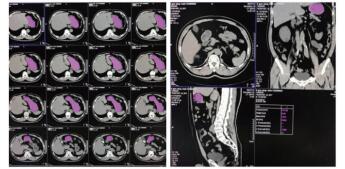
1.3 CT计算胃残余量和超声测定胃窦截面积⑴CT计算胃残余量:使用64排128层4D螺旋CT机(SOMATOM Definition AS型,德国SIEMENS公司)进行腹部CT检查,然后利用VOLUME-Work Flow医学图像软件(德国SIEMENS公司)对CT图像进行分析,即以Freehand方式对胃进行描记,从胃底开始逐层勾画胃壁轮廓形态并进行修正,以及设置CT评估限值,最终通过软件自动计算出胃腔总容积、胃腔气体容积、胃腔非气体容积及胃腔容积高度,其中胃腔非气体容积为患者胃残余量值(见图 1)。
|
| 图 1 CT检测胃容积的方法(左:描计胃轮廓;右:胃残余量计算) Fig 1 The capacity of stomach measured by CT (Left, the tracing of gastric outline; Right, the calculation of gastric residual volume) |
|
|
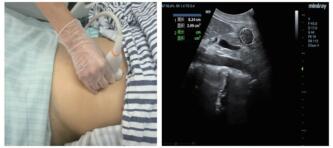
⑵超声测定截面积:由一位接受过急危重症超声规范培训的医师完成。使用M9便携式彩色多普勒超声诊断仪(深圳迈瑞公司),选择低频曲阵探头、频率2~5 MHz、标准腹部模式,患者分别于半坐位、平卧位与右侧卧位三种体位,探头置于上腹部矢状位或旁矢状位扫描,以腹主动脉、肠系膜上动静脉为定位标志获取胃窦图像,应用自由追踪描记技术描记胃窦截面的轮廓范围,取三次测量数据的平均值后计算胃窦截面积(见图 2)。
|
| 图 2 床旁超声检测胃窦截面积的方法(左:探头位置;右:胃窦截面轮廓描记) Fig 2 The cross-sectional area of gastric antrum measured by bedside ultrasound (Left, probe position; Right, outline tracing of gastric antrum cross-section) |
|
|
肠内营养不耐受的判断方法和标准:回顾分析所有入选患者的临床数据资料,结合喂养不耐受的定义界定患者是否发生肠内营养不耐受。喂养不耐受的定义为肠内营养实施期间,当营养物质连续滴注≥6 h时,患者胃残余量检测值> 250 mL或出现腹胀、腹泻、呕吐或反流等不适症状[2]。
1.4 统计学处理使用SPSS 18.0统计软件(IBM公司,美国)进行统计学分析。正态分布的计量资料以均数±标准差(Mean±SD)表示,非正态分布的计量资料以中位数和四分位数间距[M(P25, P75)]表示。采用Pearson相关分析法评估胃窦截面积与胃残余量之间的相关性,两个独立样本t检验比较危重患者喂养耐受者与不耐受者胃窦截面积之间的差异,以及受试者工作特征曲线(receiver operating characteristic curve, ROC)分析胃窦截面积对肠内营养喂养不耐受的预测价值。以P < 0.05为差异有统计学意义。
2 结果 2.1 一般资料研究共纳入42例危重患者,其中男性31例、女性11例,年龄为(53±13)岁,平均体质量为(60±8)kg,平均身高为(167±7)cm,平均体质量指数为(21.5±2.8)kg/m2。患者疾病包括多发伤20例、颅脑损伤10例、心脏骤停2例、重症胰腺炎2例、农药中毒2例、脓毒症2例、其他4例,GCS评分中位数值为15.0(5.8, 15.0),平均APACHE Ⅱ评分为(17.0±6.9)。
2.2 胃窦截面积与胃残余量的相关性分析腹部CT检测显示患者胃残余量值为(314.5±126.6)mL。床旁超声检测显示患者在半坐位、平卧位及右侧卧位等三种体位时的胃窦截面积值分别为(7.11±4.13)cm2、(4.22±2.66)cm2、(8.36±4.58)cm2。相关性分析显示,胃残余量与半坐位胃窦截面积呈显著正相关(r=0.543,P < 0.001),与平卧位胃窦截面积呈显著正相关(r=0.604,P < 0.001),与右侧卧位胃窦截面积亦呈显著正相关(r=0.618,P < 0.001)。
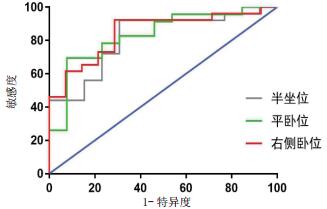
2.3 胃窦截面积对肠内营养喂养不耐受的预测价值根据喂养不耐受诊断标准,本研究危重患者在肠内营养期间喂养耐受者27例、喂养不耐受者15例。其中,喂养不耐受者在半坐位、平卧位及右侧卧位时的胃窦截面积值分别为(8.53±4.07)cm2、(5.15±2.75)cm2、(10.32±4.06)cm2,喂养耐受者在相应体位时的胃窦截面积值分别为(4.60±2.76)cm2、(2.61±1.32)cm2、(4.95±3.20)cm2;与喂养耐受者相比,喂养不耐受者三种体位胃窦截面积值均显著增加,组间比较差异均有统计学意义[均P < 0.005]。ROC分析显示,危重患者在半坐位检测时,胃窦截面积预测喂养不耐受的ROC曲线下面积为0.815,以≥3.917 cm2为截点时的敏感性为92.0%、特异性为69.2%;在平卧位检测时,胃窦截面积预测喂养不耐受的ROC曲线下面积为0.833,以≥3.395 cm2为截点时的敏感性为69.6%、特异性为92.3%;在右侧卧位检测时,胃窦截面积预测喂养不耐受的ROC曲线下面积为0.849,以≥4.402 cm2为截点时的敏感性为92.3%、特异性为71.4%。见图 3。
|
| 图 3 不同体位超声测量胃窦截面积预测喂养不耐受的ROC曲线 Fig 3 ROC curves of gastric antrum cross-sectional area in predicting feeding intolerance by ultrasound under different body positions |
|
|
CU危重患者可因各种应激因素导致胃肠组织灌注与分泌不足、胃肠动力下降、肠道微生物及宿主免疫调节紊乱等异常,能造成高达59.1%的患者发生胃肠功能障碍[7-10]。胃潴留作为胃肠功能障碍的最常见表现形式,可继发胃内容物反流与误吸,不仅影响营养物质的吸收与利用,还可导致吸入性肺炎的发生,最终恶化患者的临床预后结局[1-2]。因而,加强危重患者的胃容量监测,降低胃潴留及其并发症的发生,是ICU患者救治的重要内容之一。传统的胃管抽吸法是临床普遍使用且简便易行的评估方法,但研究证实其并不能准确地监测危重患者的胃容量情况[3]。近年来,研究发现磁共振和CT是精准地检测胃容量的有效手段。Haans等[4]研究显示磁共振能准确地评估成人的胃容量状态及其进餐后胃容量的动态变化过程。Pawanindra等[5]研究发现CT是反映肥胖患者行胃减容手术前后胃容量状态的良好方法。然而,由于上述检测手段需要转运患者、费用较高、存在辐射伤害等影响因素,不便于为ICU危重患者提供实时、动态的床旁胃容量监测。当前临床仍需一种更为便捷有效的胃容量监测工具。
床旁胃超声作为一种兼具无创、价廉、实时、便捷等多种优点的技术,已逐渐被证实能有效地监测各类手术患者的胃容量状态。Okada等[11]选择39例急诊腹部外科手术患者,发现患者在术前平卧位下超声检测获得的胃窦截面积与CT检测的胃容量值呈显著正相关,胃窦截面积能预测患者术中出现胃内容物反流误吸的风险,其敏感性与特异性分别达到85%和53%。Sharma等[12]选择100例行择期手术的外科患者,发现患者在术前进行右侧卧位胃超声检查,所获得的胃容量值能鉴别术前6~10 h禁食的有效性,尤其是发现那些可能因糖尿病、肥胖、慢性肾病等疾病而出现高几率误吸危险的患者。本研究将床旁胃超声技术用于危重患者胃残余量的监测,选择半坐位、平卧位及右侧卧位等三种体位,评估胃窦截面积反映胃残余量的准确性。结果显示,危重患者在三种体位下经床旁超声检测获得的胃窦截面积均与腹部CT测的胃残余量呈正相关,其中右侧卧位下的超声检测值的相关性最强(r=0.618,P < 0.001)。因而,床旁胃超声可能成为替代磁共振与CT为危重患者进行胃残余量监测的有力手段。
肠内营养不仅能为危重患者补充足够的营养物质,还利于维护胃肠道结构与功能的完整性、降低肠道渗透性及改善免疫系统功能等,进而促进胃肠功能障碍的恢复,已成为危重患者的重要治疗[13]。目前的营养治疗指南建议,危重患者若不存在肠内营养治疗的禁忌,应在入院24~48 h内实施肠内营养支持治疗[14]。然而,危重患者的胃肠功能障碍可能导致肠内营养期间喂养不耐受的发生,进而增加胃潴留甚至反流、误吸的风险。因而,在肠内营养实施的过程中,需要进行胃残余量监测,以实时辅助评估患者喂养不耐受的发生及进行肠内营养治疗方案的调整,为患者最大程度地实现充分的肠内营养治疗。本研究发现床旁超声能较为准确地反应胃残余量,在此基础上进一步分析了其对肠内营养喂养不耐受的预测价值。结果显示,危重患者在半坐位、平卧位及右侧卧位等三种体位下,进行超声检测获得的胃窦截面积预测肠内营养喂养不耐受的ROC曲线下面积分别为0.815、0.833、0.849,提示三种体位下的检测结果均能预测喂养不耐受的发生,其中右侧卧位下检测值的敏感性与特异性最佳,预测效果最好。因而,床旁胃超声检测可作为危重患者肠内营养喂养不耐受的有效预测工具。
本研究也存在一些局限性。首先,床旁胃超声检查易受患者体型、体位及胃肠道气体干扰等因素的影响,可能造成部分患者不能获得准确的胃窦截面积值。其次,胃超声检查人员的操作熟练程度及技术水平可能造成测量结果出现一定程度的偏差。第三,回顾性研究提供的信息有限。综上所述,床旁胃超声能比较准确地评估危重患者的胃残余量,并能有效地预测肠内营养期间喂养不耐受的发生,有利于指导肠内营养治疗的调整与优化。
利益冲突 所有作者均声明不存在利益冲突
| [1] | Elke G, Felbinger TW, Heyland DK. Gastric residual volume in critically ill patients: a dead marker or still alive[J]. Nutr Clin Pract, 2015, 30(1): 59-71. DOI:10.1177/0884533614562841 |
| [2] | Blaser AR, Starkopf J, Kirsimägi ü, et al. Definition, prevalence, and outcome of feeding intolerance in intensive care: a systematic review and meta-analysis[J]. Acta Anaesthesiol Scand, 2014, 58(8): 914-922. DOI:10.1111/aas.12302 |
| [3] | Bouvet L, Zieleskiewicz L, Loubradou E, et al. Reliability of gastric suctioning compared with ultrasound assessment of residual gastric volume: a prospective multicentre cohort study[J]. Anaesthesia, 2019, Dec 4. DOI:10.1111/anae.14915 |
| [4] | Haans JJ, de Zwart IM, Eilers PH, et al. Gastric volume changes in response to a meal: validation of magnetic resonance imaging versus the barostat[J]. J Magn Reson Imaging, 2011, 34(3): 685-690. DOI:10.1002/jmri.22619 |
| [5] | Pawanindra L, Vindal A, Midha M, et al. Early post-operative weight loss after laparoscopic sleeve gastrectomy correlates with the volume of the excised stomach and not with that of the sleeve! Preliminary data from a multi-detector computed tomography-based study[J]. Surg Endosc, 2015, 29(10): 2921-2927. DOI:10.1007/s00464-014-4021-9 |
| [6] | 叶秋萍, 陆姚, 方卫萍. 床旁胃部超声在评估反流误吸中的应用进展[J]. 国际麻醉学与复苏杂志, 2019, 40(9): 875-880. DOI:10.3760/cma.j.issn.1673-4378.2019.09.016 |
| [7] | Reintam Blaser A, Malbrain ML, Starkopf J, et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems[J]. Intensive Care Med, 2012, 38(3): 384-394. DOI:10.1007/s00134-011-2459-y |
| [8] | Chapman MJ, Nguyen NQ, Deane AM. Gastrointestinal dysmotility: evidence and clinical management[J]. Curr Opin Clin Nutr Metab Care, 2013, 16(2): 209-216. DOI:10.1097/MCO.0b013e32835c1fa5 |
| [9] | Schuijt TJ, van der Poll T, de Vos WM, et al. The intestinal microbiota and host immune interactions in the critically ill[J]. Trends Microbiol, 2013, 21(5): 221-229. DOI:10.1016/j.tim.2013.02.001 |
| [10] | Reintam A, Parm P, Kitus R, et al. Gastrointestinal symptoms in intensive care patients[J]. Acta Anaesthesiol Scand, 2009, 53(3): 318-324. DOI:10.1111/j.1399-6576.2008.01860.x |
| [11] | Okada Y, Toyama H, Kamata K, et al. A clinical study comparing ultrasound-measured pyloric antrum cross-sectional area to computed tomography-measured gastric content volume to detect high-risk stomach in supine patients undergoing emergency abdominal surgery[J]. J Clin Monit Comput, 2019, Dec 7. DOI:10.1007/s10877-019-00438-1 |
| [12] | Sharma G, Jacob R, Mahankali S, et al. Preoperative assessment of gastric contents and volume using bedside ultrasound in adult patients: A prospective, observational, correlation study[J]. Indian J Anaesth, 2018, 62(10): 753-758. DOI:10.4103/ija.IJA_147_18 |
| [13] | McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition[J]. Nutr Clin Pract, 2009, 24(3): 305-315. DOI:10.1177/0884533609335176 |
| [14] | McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.)[J]. JPEN J Parenter Enteral Nutr, 2016, 40(2): 159-211. DOI:10.1177/0148607115621863 |
 2020, Vol. 29
2020, Vol. 29




