脓毒症是由宿主对感染的反应失调引起的危及生命的器官功能障碍,是急危重症医学领域的重要临床问题,具有高发病率、高病死率的特点[1-2]。肺脏是脓毒症期间最易损伤的器官之一,通常会发展为急性肺损伤(acute lung injury,ALI)。尽管针对ALI的研究较多,但其具体机制尚不完全清楚,其中炎症介质和细胞凋亡在脓毒症ALI的发病机制研究中占据重要地位[3-4]。Mitofusin(Mfn)2是线粒体融合和分裂的关键分子之一[5]。有研究发现Mfn2基因突变引起线粒体分裂融合稳态不平衡,这种稳态不平衡不仅导致神经退行性疾病,还将导致细胞炎症,氧化应激和细胞凋亡的发生[6]。姜黄素是姜黄的主要活性成分之一,已被用作色素、食品添加剂和调味品等[7]。已有实验研究表明,用姜黄素预处理可以减少组织损伤并提高脓毒症小鼠的存活率,姜黄素对LPS诱导的急性肺损伤具有治疗作用, 可有效治疗或控制炎症,氧化应激和细胞凋亡[8-9]。姜黄素对脓毒症相关ALI的保护作用已得到证实,但其具体机制尚不清楚。本研究首先观察姜黄素对脓毒症小鼠ALI的保护作用,并且通过体内调节Mfn2进一步探究其可能机制。
1 材料与方法 1.1 试剂和试剂盒姜黄素(Sigma公司,美国);TUNEL检测试剂盒(索莱宝生物技术有限公司,北京);玉米油(太阳能生物科技有限公司,上海);凋亡试剂盒(BD公司,美国);小鼠脾脏组织淋巴细胞分离液(天津市灏洋生物制品科技有限责任公司,天津);BCA蛋白浓度测定试剂盒(Thermo Scientific公司,美国);兔抗小鼠Mfn2单抗(abcam公司,英国)兔抗小鼠caspase-3、caspase-3 cleaved、β-actin单抗、山羊抗兔二抗(Cell Signaling Technology公司,美国)。
1.2 实验动物和模型建立本研究动物实验过程遵循国际公认的动物福利和道德准则。120只健康雄性清洁级Balb/c小鼠,体质量20~ 25 g,由上海斯莱克实验动物有限公司提供,许可证号:SCXK(沪)2017-0421。小鼠育种和动物实验在温州医科大学实验动物中心进行,使用许可证号:SYXK(浙)2015-0009。120只Balb/c小鼠随机(随机数字法)分为假手术组、脓毒症组、姜黄素对照组、姜黄素干预组、阴性病毒-脓毒症组、阴性病毒-姜黄素干预组、Mfn2干扰-脓毒症组、Mfn2干扰-姜黄素干预组,每组15只。将脓毒症组的小鼠利用盲肠结扎穿孔术(CLP)建立模型,即禁食1 d,自由饮水,腹腔注射1%戊巴比妥钠10~50 mg/kg,中位切口麻醉1~2 cm,结扎盲肠在两处用21号针刺穿,并通过穿刺伤口渗漏少量盲肠内容物以诱发脓毒症。在盲肠回纳腹膜腔后,缝合肌肉层和表皮层,并给予无菌生理盐水溶液(24 mL/kg体质量)用于液体复苏,在最初的6 h内,存活率为100%,并且小鼠出现抑郁和腹泻等典型症状,表明建模成功。CLP后72 h的病死率约为40%~60%。假手术组小鼠接受剖腹术,未行结扎穿孔。姜黄素干预组及姜黄素对照组予姜黄素200 mg/(kg·d)(姜黄素按1%浓度溶于玉米油中)灌胃1周后,姜黄素干预组小鼠予同假手术组处理,而姜黄素干预组予同脓毒症组小鼠处理。腺相关病毒由上海吉凯基因化学有限公司构建,已由前期研究证实该病毒可用于后续研究[10]。阴性病毒组在CLP术前两周予小鼠尾静脉注射腺相关病毒阴性对照病毒,Mfn2干扰组在CLP术前两周予小鼠尾静脉注射携带Mfn2干扰序列的腺相关病毒转染。
1.3 小鼠肺组织湿干比的检测CLP造模24 h后,打开小鼠胸腔,取左肺吸收干燥的表面液体,称湿质量,然后将其放入80℃的烘箱中晾干至恒重,称干质量;肺组织湿干比=(湿质量-干质量)/湿质量×100%。
1.4 小鼠支气管肺泡灌洗液(bronchoalveolar lavage fluid, BALF)炎症因子的检测CLP术后24 h,将小鼠麻醉并在胸骨中间打开以暴露气管和肺。结扎右肺门,沿气管前壁插入注射器针头,注射0.5 mL冰PBS。2 min后,吸出冲洗液并再次注入肺组织。以此方法进行5次支气管肺泡灌洗,恢复率为90%。将回收的肺泡灌洗液立即置于低温高速离心机中,4 ℃500×g离心10 min,取上清液。按照试剂盒说明书,通过ELISA测量TNF-α和IL-6的浓度。
1.5 组织病理学的检测在CLP术后24 h收集小鼠左肺,置于10%中性甲醛溶液中,石蜡包埋,制备5 μm切片,并进行苏木精-伊红染色(HE)。光镜下观察肺组织病理变化。从每只小鼠的肺切片中随机选择总共10个视野。病理学专业人员分别对肺水肿、肺泡和间质炎症、肺泡和间质出血、肺不张和透明膜形成进行半定量分析。无损伤:0分,病变范围 < 25%:1分; 25%~50%:2分; 50%~70%:3分,全视野:4分。计算总得分并进行评估。
1.6 通过TUNEL法测定检测细胞凋亡采用TUNEL法按试剂盒说明操作进行。取小鼠肺脏后以4%多聚甲醛固定,脱水后石蜡包埋制备石蜡切片,二甲苯脱蜡,梯度酒精水化后,PBS冲洗2次,每次3 min;蛋白酶K消化15 min后,PBS冲洗2次,每次3 min;擦干组织周围的水份,每片加50 μL的0.3% H2O2甲醇溶液,阻断内源性过氧化酶,PBS冲洗2次,每次3 min; 擦干组织周围的水份。每片加25 μL的TUNEL反应液,于孵育盒中37 ℃,孵育60 min,PBS冲洗3次,每次3 min;擦干组织周围的水份,每片加25 μL的辣根过氧化酶抗体,孵育盒中37 ℃,孵育30 min,PBS冲洗3次,每次3 min; 擦干组织周围的水份,每片加一滴新鲜配制的DAB室温孵育5~10 min,PBS冲洗3次,每次3 min。苏木精复染,酒精脱水,干燥,中性树胶封片。细胞核呈棕色的细胞为凋亡阳性细胞。每组随机取10张切片,每张切片在高倍镜下(×400)随机观察5个不重叠视野,计算阳性细胞数和总细胞数的比值,取其平均数为凋亡指数(apoptotic index, AI),然后对其凋亡指数进行评分,0~1%为0分、1%~10%为1分、10%~50%为2分、50%~80%为3分、80%~100%为4分。
1.7 肺组织中caspase-3和caspase-3 cleaved蛋白表达的检测收集细胞后,用预冷的裂解缓冲液裂解细胞,用超声波细胞破碎器破碎细胞3次,然后在4℃以14 000 r/min离心20 min。使用BCA(二喹啉甲酸)法测量上清液中的蛋白质浓度后,将蛋白质与合适的SDS上样缓冲液混合,然后在95 ℃下煮沸5 min。将蛋白质在12% SDS-PAGE凝胶中分离,然后转移到聚偏二氟乙烯膜(PVDF)中。在室温下用5 %脱脂乳封闭2 h后,用抗caspase-3(1 : 1 000),抗caspase cleaved(1 : 1 000)和抗β-肌动蛋白(1 : 1 000)抗体4 ℃孵育过夜。然后,加入辣根过氧化物酶-(HRP)缀合的山羊抗兔二抗(1 : 5 000)在室温下孵育1 h。用TBST洗涤后,使用ECL显影并使用图像分析系统分析条带灰度值。
1.8 统计学方法应用SPSS 23.0软件进行统计分析,计量资料若服从正态分布则以均数±标准差(Mean±SD)表示,两组样本比较采用独立样本t检验,多组均数比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果 2.1 各种小鼠肺湿/干比与假手术组(31.11±5.60)%相比,脓毒症组(71.11±3.78)%的小鼠肺脏湿/干比显著增加,差异有统计学意义(t =6.798,P= 0.002)。CLP术前姜黄素预处理(32.84±6.15)%与脓毒症组比较明显下降,差异有统计学意义(t =6.125,P= 0.004),提示姜黄素对脓毒症相关ALI有明显的保护作用。阴性病毒干预后,姜黄素干预组(32.29±3.67)%较脓毒症组(61.96±3.32)%小鼠肺脏湿/干比明显降低,差异有统计学意义(t =5.999,P= 0.004);当通过腺相关病毒给予下调Mfn2后,姜黄素干预组(55.95±4.35)%较脓毒症组(54.24±4.86)%差异无统计学意义(P > 0.05),见图 1。
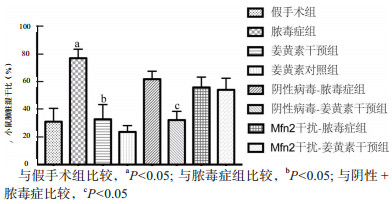
|
| 图 1 姜黄素对脓毒症小鼠肺组织湿/干比的影响 Fig 1 Effect of curcumin on W/D of lung tissue in septic mice |
|
|
Western bolt结果显示脓毒症组caspase-3蛋白表达显著低于假手术组(P= 0.001),而caspase-3 cleaved则相反,说明脓毒症期间caspase-3被激活。姜黄素预处理后caspase-3蛋白的表达较脓毒症组明显上调(P= 0.001),说明姜黄素干预可预防caspase-3被激活而引发细胞凋亡的发生。当通过腺相关病毒下调Mfn2的基因表达后,Mfn2干扰-姜黄素干预组的caspase-3的蛋白表达较Mfn2干扰-脓毒症组差异无统计学意义(P= 0.005),见图 2。
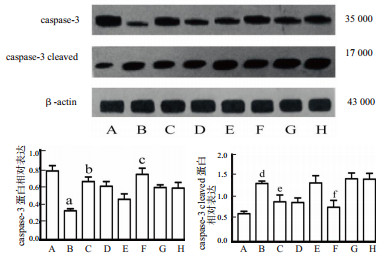
|
| A:假手术组;B:脓毒症组;C:姜黄素对照组;D:姜黄素干预组;E:阴性病毒-脓毒症组;F:阴性病毒-姜黄素干预组;G:Mfn2干扰-脓毒症组;H:Mfn2干扰-姜黄素干预组;与假手术组比较,aP < 0.05;与脓毒症组比较,bP < 0.05;与阴性病毒+脓毒症比较,cP < 0.05;与假手术组比较,dP < 0.05;与脓毒症组比较,eP < 0.05;与阴性病毒+脓毒症组比较,fP < 0.05 图 2 姜黄素对脓毒症小鼠caspase-3和caspase-3 cleaved蛋白表达的影响 Fig 2 Effect of curcumin on the expression of caspase-3 and caspase-3 cleaved protein |
|
|
姜黄素对脓毒症肺部炎症及损伤影响的病理学结果显示,与假手术组相比,脓毒症组肺组织结构不完整,肺泡隔水肿,炎症,肺泡腔不明显,而姜黄素干预组上述病变明显减轻。为了进一步探索这种机制,下调Mfn2基因后观察姜黄素干预对脓毒症的保护作用(图 3)。病理评分显示,阴性病毒-姜黄素干预组较脓毒症组病理评分显著降低,差异有统计学意义(P < 0.05),Mfn2干扰-姜黄素干预组较脓毒症组的病理评分差异无统计学意义(P > 0.05)。
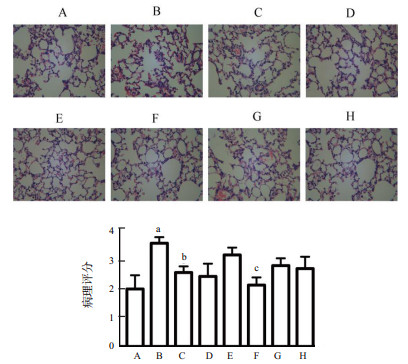
|
| A:假手术组;B:脓毒症组;C:姜黄素对照组;D:姜黄素干预组;E:阴性病毒-脓毒症组;F:阴性病毒-姜黄素干预组;G:Mfn2干扰-脓毒症组;H:Mfn2干扰-姜黄素干预组;与假手术组比较,aP < 0.05;与脓毒症组比较,bP < 0.01;与阴性病毒-脓毒症组比较,cP < 0.05 图 3 姜黄素对脓毒症急性肺损伤影响的病理学观察(n=3)(×400) Fig 3 Pathological observation of the effect of curcumin on acute lung injury in sepsis (n=3) (×400) |
|
|
采用TUNEL法检测小鼠肺脏细胞凋亡情况,与假手术组凋亡指数相比,脓毒症组细胞凋亡指数显著增高,而姜黄素干预组较脓毒症组的凋亡指数明显降低。进一步下调Mfn2后,Mfn2干扰-姜黄素组与Mfn2干扰-脓毒症组比较未发现凋亡指数明显降低。见图 4。
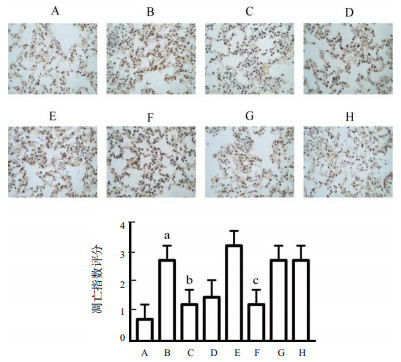
|
| A:假手术组;B:脓毒症组;C:姜黄素对照组;D:姜黄素干预组;E:阴性病毒-脓毒症组;F:阴性病毒-姜黄素干预组;G:Mfn2干扰-脓毒症组;H:Mfn2干扰-姜黄素干预组;与假手术组比较,aP < 0.05;与脓毒症组比较,bP < 0.01;与阴性病毒-脓毒症组比较,cP < 0.05 图 4 各组肺组织细胞凋亡(TUNEL×400) Fig 4 Apoptosis of lung tissue in each group (TUNEL×400) |
|
|
ELISA显示脓毒症组肺泡灌洗液中炎症因子TNF-α和IL-6水平显著高于假手术组,差异均有统计学意义(P < 0.05)。与脓毒症组相比,姜黄素干预组TNF-α和IL-6表达水平明显降低(P < 0.05)。在下调Mfn2的基因后,姜黄素对脓毒症的作用显著降低甚至消失,见表 1。
| 组别 | TNF-α | IL-6 |
| 假手术组 | 35.47±0.42 | 41.95±2.82 |
| 脓毒症组 | 72.27±1.04 a | 66.94±10.76 d |
| 姜黄素干预组 | 48.45±10.95 b | 47.79±6.65 e |
| 姜黄素对照组 | 27.69±3.53 | 38.43±9.68 |
| 阴性病毒-脓毒症组 | 56.22±3.40 | 71.86±2.42 |
| 阴性病毒-姜黄素组 | 29.05±2.35 c | 44.76±3.63 f |
| Mfn2干预-脓毒症组 | 46.94±4.49 | 60.63±11.41 |
| Mfn2干预-姜黄素组 | 49.17±3.57 | 63.34±6.04 |
| 注:与假手术组比较,a P < 0.05;与脓毒症组比较,b P < 0.01;与阴性病毒-脓毒症组比较,c P < 0.05;与假手术组比较,d P < 0.05;与脓毒症组比较,e P < 0.05;与阴性病毒-脓毒症组比较,f P < 0.05;TNF-α为肿瘤坏死因子-α;IL-6为白介素-6 | ||
本研究旨在探讨姜黄素对脓毒症相关急性肺损伤的保护作用是否与小鼠中盲肠结扎穿孔术诱导的多微生物型脓毒症模型的Mfn2表达密切相关。盲肠结扎穿孔术式目前被认为是脓毒症的金标准模型,它是最广泛使用的模型,因为它与人类脓毒症的进展和病理生理学非常相似[11-12]。研究发现,在造模术后24 h小鼠脓毒症症状最明显,因此本研究脓毒症诱导的ALI小鼠的功能和结构时选取CLP术后24 h [13]。腺相关病毒是一类单链线状DNA缺陷型病毒,是一种辅助依赖性细小病毒,与人类疾病无关,该方面结合其广泛的细胞和组织趋向性以及有限的病毒宿主反应使其成为干扰基因有吸引力的载体系统[14]。腺相关病毒介导的基因治疗已经在癌症方面有多项研究[15-16]。该病毒已发展成为最重要和最安全的病毒之一[17]。因此选择腺相关病毒作为载体,构建携带Mfn2干扰序列的腺相关病毒后,通过尾静脉注射Balb/c小鼠构建Mfn2下调的小鼠模型。
姜黄素是天然产物姜黄的主要成分[18]。最近的一些实验表明,用姜黄素预处理可以减少脓毒症小鼠组织损伤并增加小鼠的存活率。虽然其生物利用度较低,但是由于具有抗炎、抗氧化、降血脂、清除自由基、抗肿瘤、抑菌等多种药理活性而得到广泛的应用。已有研究证明姜黄素对LPS诱导的急性肺损伤具有治疗益处。TNF-α和IL-6是治疗许多肺部炎症性疾病(包括急性肺损伤)的关键调节剂[19-20]。本研究结果显示,TNF-α和IL-6的表达可以在脓毒症中明显上调,并可在姜黄素预处理后下降。此外,组织病理学检查显示脓毒症期间肺组织病理切片结构明显不完整,肺泡腔内有大量浆液性渗出物,混有少量白细胞、红细胞和纤维素性渗出物;而在姜黄素干预后,肺组织结构较为完整,肺泡腔内有少量的渗出物,这些结果强提示姜黄素在脓毒症相关ALI中的保护作用。
脓毒症诱导ALI的病理生理学的特征涉及炎症、细胞因子、趋化因子以及凋亡激活剂和抑制剂等复杂机制[21-23]。Mfn2是治疗许多疾病的靶基因,Mfn2的正常表达与细胞炎症,凋亡及能量代谢等方面息息相关[24]。Mfn2也是维持线粒体融合分裂稳态的关键蛋白之一,这种稳态对于细胞有起到保护作用[25]。目前已经进行了许多研究来探讨Mfn2在脓毒症中的作用,但Mfn2与脓毒症相关的ALI目前尚未有深入研究[26]。Muñoz等[27]研究表明Mfn2缺陷细胞引起大量内质网(ER)扩张,称为ER应激,会导致caspase-3激活,启动细胞凋亡。因此通过携带Mfn2干扰的腺相关病毒下调小鼠中的Mfn2序列。本研究结果表明,脓毒症期间,小鼠肺组织的caspase-3会被激活,转变为caspase-3 cleaved状态,同时肺组织湿干比增加,说明肺损伤加重,姜黄素的干预会预防caspase-3激活,降低肺组织湿干比。当Mfn2被下调时,Mfn2干扰-脓毒症组和Mfn2干扰-姜黄素组之间caspase-3的表达水平差异明显减少,姜黄素的干预后caspase-3仍有被激活,诱导细胞凋亡的发生,姜黄素干预后湿干重比也较脓毒症组无明显变化。因此,笔者推测姜黄素对脓毒症的影响是依赖于Mfn2。
综上所述,姜黄素可通过降低肺组织湿干比,减轻肺组织病理损伤,降低肺泡灌洗液中炎症因子TNF-α和IL-6的表达,减少肺组织细胞凋亡,从而减轻脓毒症小鼠急性肺损伤,其可能机制是通过上调Mfn2的表达来实现。
利益冲突 所有作者均声明不存在利益冲突
| [1] | SepNet Critical Care Trials Group. Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study[J]. Intensive Care Med, 2016, 42(12): 1980-1989. DOI:10.1007/s00134-016-4504-3 |
| [2] | Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3)[J]. JAMA, 2016, 315(8): 801-810. DOI:10.1001/jama.2016.0287 |
| [3] | Sadowitz B, Roy S, Gatto LA, et al. Lung injury induced by sepsis: lessons learned from large animal models and future directions for treatment[J]. Expert Rev Anti Infect Ther, 2011, 9(12): 1169-1178. DOI:10.1586/eri.11.141 |
| [4] | Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis[J]. Nat Med, 2012, 18(8): 1217-1223. DOI:10.1038/nm.2843 |
| [5] | Sevransky JE, Martin GS, Shanholtz C, et al. Mortality in sepsis versus non-sepsis induced acute lung injury[J]. Crit Care, 2009, 13(5): R150. DOI:10.1186/cc8048 |
| [6] | Gill SE, Rohan M, Mehta S. Role of pulmonary microvascular endothelial cell apoptosis in murine sepsis-induced lung injury in vivo[J]. Respir Res, 2015, 16: 109. DOI:10.1186/s12931-015-0266-7 |
| [7] | Lin WC, Chen CW, Huang YW, et al. Kallistatin protects against sepsis-related acute lung injury via inhibiting inflammation and apoptosis[J]. Sci Rep, 2015, 5: 12463. DOI:10.1038/srep12463 |
| [8] | Xiao X, Yang M, Sun D, et al. Curcumin protects against sepsis-induced acute lung injury in rats[J]. J Surg Res, 2012, 176(1): e31-39. DOI:10.1016/j.jss.2011.11.1032 |
| [9] | Yu J, Shi J, Wang D, et al. Heme oxygenase-1/carbon monoxide-regulated mitochondrial dynamic equilibrium contributes to the attenuation of endotoxin-induced acute lung injury in rats and in lipopolysaccharide-activated macrophages[J]. Anesthesiology, 2016, 125(6): 1190-1201. DOI:10.1097/ALN.0000000000001333 |
| [10] | 支绍册, 郑来赞, 洪广亮, 等. 线粒体融合蛋白2在姜黄素减轻脓毒症小鼠淋巴细胞凋亡中的作用[J]. 浙江医学, 2019, 41(18): 1921-1927. DOI:10.12056/j.issn.1006-2785.2019.41.18.2019-801 |
| [11] | Franco A, Kitsis RN, Fleischer JA, et al. Correcting mitochondrial fusion by manipulating mitofusin conformations[J]. Nature, 2016, 540(7631): 74-79. DOI:10.1038/nature20156 |
| [12] | Das N, Mandala A, Naaz S, et al. Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice[J]. J Pineal Res, 2017, 62(4). DOI:10.1111/jpi.12404 |
| [13] | Marchiani A, Rozzo C, Fadda A, et al. Curcumin and curcumin-like molecules: from spice to drugs[J]. Curr Med Chem, 2014, 21(2): 204-222. DOI:10.2174/092986732102131206115810 |
| [14] | Edwards RL, Luis PB, Varuzza PV, et al. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites[J]. J Biol Chem, 2017, 292(52): 21243-21252. DOI:10.1074/jbc.RA117.000123 |
| [15] | He Y, Yue Y, Zheng X, et al. Curcumin, inflammation, and chronic diseases: how are they linked?[J]. Molecules, 2015, 20(5): 9183-9213. DOI:10.3390/molecules20059183 |
| [16] | Xu F, Lin SH, Yang YZ, et al. The effect of curcumin on sepsis-induced acute lung injury in a rat model through the inhibition of the TGF-beta1/SMAD3 pathway[J]. Int Immunopharmacol, 2013, 16(1): 1-6. DOI:10.1016/j.intimp.2013.03.014 |
| [17] | Dejager L, Pinheiro I, Dejonckheere E, et al. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis?[J]. Trends Microbiol, 2011, 19(4): 198-208. DOI:10.1016/j.tim.2011.01.001 |
| [18] | Zhang Z, Zhou J, Liao C, et al. RAGE deficiency attenuates the protective effect of Lidocaine against sepsis-induced acute lung injury[J]. Inflammation, 2017, 40(2): 601-611. DOI:10.1007/s10753-016-0507-z |
| [19] | Rittirsch D, Huber-Lang MS, Flierl MA, et al. Immunodesign of experimental sepsis by cecal ligation and puncture[J]. Nat Protoc, 2009, 4(1): 31-36. DOI:10.1038/nprot.2008.214 |
| [20] | Nelson KM, Dahlin JL, Bisson J, et al. The essential medicinal chemistry of curcumin[J]. J Med Chem, 2017, 60(5): 1620-1637. DOI:10.1021/acs.jmedchem.6b00975 |
| [21] | Zhu H, Xu T, Qiu C, et al. Synthesis and optimization of novel allylated mono-carbonyl analogs of curcumin (MACs) act as potent anti-inflammatory agents against LPS-induced acute lung injury (ALI) in rats[J]. Eur J Med Chem, 2016, 121: 181-193. DOI:10.1016/j.ejmech.2016.05.041 |
| [22] | Malaviya R, Laskin JD, Laskin DL. Anti-TNFalpha therapy in inflammatory lung diseases[J]. Pharmacol Ther, 2017, 180: 90-98. DOI:10.1016/j.pharmthera.2017.06.008 |
| [23] | Zhang H, Neuhofer P, Song L, et al. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality[J]. J Clin Invest, 2013, 123(3): 1019-1031. DOI:10.1172/JCI64931 |
| [24] | 刘雪梅, 孟庆华. 线粒体融合蛋白-2研究进展[J]. 北京医学, 2011, 33(6): 497-501. DOI:10.15932/j.0253-9713.2011.06.008 |
| [25] | 连洁, 洪广亮, 仝欢, 等. 姜黄素上调线粒体融合蛋白2抑制脓毒症小鼠淋巴细胞凋亡的实验研究[J]. 中华急诊医学杂志, 2016, 25(2): 153-159. DOI:10.3760/cma.j.issn.1671-0282.2016.02.005 |
| [26] | Ying L, Zhao GJ, Wu Y, et al. Mitofusin 2 promotes apoptosis of CD4(+) T cells by inhibiting autophagy in sepsis[J]. Mediators Inflamm, 2017, 2017: 4926205. DOI:10.1155/2017/4926205 |
| [27] | Muñoz JP, Ivanova S, Sanchez-Wandelmer J, et al. Mfn2 modulates the UPR and mitochondrial function via repression of PERK[J]. EMBO J, 2013, 32(17): 2348-2361. DOI:10.1038/emboj.2013.168 |
 2020, Vol. 29
2020, Vol. 29




