2 天津市第一中心医院 重症医学科,300192;
3 天津市急救医学研究所,300192
2 Department of Critical Care Medicine, Tianjin First Center Hospital, Tianjin, 300192, China;
3 Tianjin First Center Hospital Emergency Medical Research Institute, Tianjin, 300192, China
脓毒症相关血小板减少症(thrombocytopenia,TCP)是脓毒症期间发生的影响生存预后及治疗效果的重要因素[1]。探索有效地纠正血小板减少状态,调节血小板功能的方法,能够改善患者的病情及预后。重组人血小板生成素(recombinant human thrombopoietin,rhTPO)可以促进化疗后骨髓抑制患者和特发性血小板减少性紫癜(idiopathic thrombocytopenic purpura, ITP)患者的血小板恢复,缩短血小板减少持续的时间[2-3]。研究表明rhTPO能够有效升高脓毒症引起的血小板减少水平,缓解脓毒症引起的TCP,但其机制尚未明确[4]。本研究以小鼠为研究对象,腹腔注射大肠杆菌脂多糖(lipopolysaccharide,LPS)制备TCP模型,不同剂量rhTPO干预后观察各组小鼠变化,为rhTPO在临床的应用提供理论依据。
1 材料与方法 1.1 材料LPS(Sigma),rhTPO(沈阳三生公司),PE标记CD61和APC标记CD62p抗体(Biolegend),GSDMD抗体(Abcam),FAM FLICA Caspsae-1 KIT(Biorad),小鼠IL-1β、IL-18 ELISA试剂盒(博士德),动物血细胞计数仪(Sysmex),台式低速离心机(Sigma),流式细胞仪(BD),显微镜(奥林巴斯)。
1.2 实验动物分组及模型制备本研究采用100只SPF级,周龄6~8周,体质量在18~22 g的雄性C57BL/6小鼠,动物合格证号为SCXK(京)2014-0004。按随机数字表法将小鼠分为5组,即空白对照组(Sham组):腹腔及皮下注射同等剂量生理盐水; 实验对照组(LPS组)LPS+生理盐水; LPS+低剂量rhTPO 1.35×103 U·kg-1·d-1组(L组)、LPS+中剂量rhTPO 2.7×103 U·kg-1·d-1组(M组)、LPS+高剂量rhTPO 5.4×103 U·kg-1·d-1组(H组)。LPS以生理盐水溶解,予小鼠单次腹腔注射30 mg/kg制备TCP脓毒症模型[5]。药物干预方法采取每隔24 h皮下注射一次,并于造模后立即开始。实验连续观察72 h。
1.3 检测指标及方法 1.3.1 小鼠血常规检测实验开始后每间隔24 h小鼠目内眦取血30 μL至抗凝离心管中,于采血4 h内动物血细胞计数仪检测血小板计数。
1.3.2 小鼠洗涤血小板的制备实验开始第72小时腹腔注射5%水合氯醛麻醉小鼠,经摘眼球取血,后处死小鼠。制备富血小板血浆,枸橼酸-葡萄糖-氯化钠缓冲液、无钙台式缓冲液洗涤,冲悬血小板,调整血小板终浓度为1×106/mL,以备流式细胞检测。
1.3.3 流式细胞技术检测CD61/CD62p阳性表达率、GSDMD、Caspse-1的表达水平取小鼠洗涤血小板,备阴性对照管、单阳性管和双阳管,分别加入相应抗体后,室温避光孵育30 min,1 mL 1%多聚甲醛固定终止反应,PBS洗涤,PBS冲悬上机。GSDMD蛋白检测使方法同前。Caspse-1的检测严格依据说明书进行。
1.3.4 ELISA技术检测小鼠血浆IL-1β、IL-18水平取小鼠富血小板血浆20 μL,ELISA操作步骤严格按照试剂盒说明书进行。
1.4 统计学方法应用SPSS 25.0软件进行统计,计量资料以均数±标准差(Mean±SD)表示,采用单因素方差分析进行组间均数两两比较,采用LSD-t检验进行两样本均数比较,通过GraphPad Prism 7.0软件绘制小鼠72 h Kaplan-Meier生存曲线,采用Log-rank检验。P<0.05为差异有统计学意义。
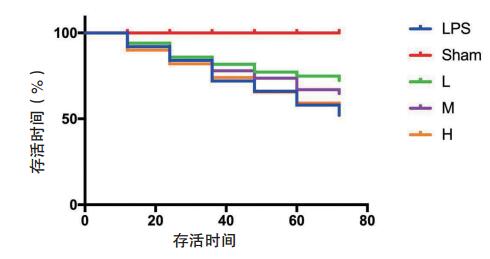
2 结果 2.1 生存曲线分析Sham组72 h生存率为100%;LPS组24 h、48 h、72 h生存率分别为84%,66%,52%;L组24 h、48 h、72 h生存率分别为85%,77%,72%;M组24 h、48 h、72 h生存率分别为84%,73%,64%;H组24 h、48 h、72 h生存率分别为82%,65%,54%。结果显示,LPS组与Sham组相比生存率明显降低,差异有统计学意义(χ2=25.03,P<0.01)。L、M、H组与LPS组相比生存率略有升高,但差异无统计学意义(P > 0.05)。见图 1。
|
| LPS组与Sham组相比生存率明显降低(χ2=25.03,P<0.01)。L、M、H组与LPS组相比差异无统计学意义(P > 0.05) 图 1 各组小鼠生存曲线的比较 Fig 1 Comparison of survival curve of mice in each group |
|
|
Sham组小鼠血小板计数在实验前后无明显变化,注射LPS后小鼠血小板计数开始下降,L组小鼠血小板计数在造模第24小时,较M组、H组、LPS组相比较无明显变化(P > 0.05),而在第48~72 h,L组小鼠血小板明显高于M组、H组及LPS组(P< 0.01),M组、H组较LPS组则无明显升高(P > 0.05)。见表 1。
| 组别 | Sham组 | LPS组 | L组 | M组 | H组 |
| 0 h | 1155.657±40.782 | 1148.718±33.300 | 1140.162±21.152 | 1126.230±39.491 | 1138.788±40.548 |
| 24 h | 1144.793±34.166 | 253.310±21.488 a | 269.444±26.889 a | 256.399±34.476 a | 247.635±31.548 a |
| 48 h | 1151.643±16.356 | 371.329±31.152 a | 586.033±90.431 a, b | 336.591±27.409 a, c | 327.076±37.269 a, c |
| 72 h | 1138.255±84.001 | 561.704±17.349 a | 925.641±79.394 c | 575.632±22.104 a, d | 553.581±44.223 a, d |
| 注:与Sham组比较,aP< 0.001;与LPS组比较,bP< 0.05,bP< 0.001;与L组比较,dP< 0.001 | |||||
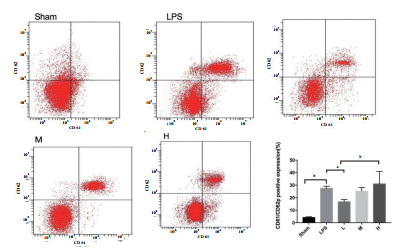
LPS组小鼠洗涤血小板CD61/CD62p表达较Sham组明显升高[(27.43 ± 0.87) vs (4.08 ± 0.35), P= 0.016],予rhTPO干预后L组CD61/CD62p表达较LPS组明显下降[(16.66 ± 0.93) vs (27.43 ± 0.87),P= 0.044],M、H组较LPS组变化不明显[(25.22 ± 1.46)、(30.92 ± 5.03) vs (27.43 ± 0.87), P= 0.965,P= 0.0841]。见图 2。
|
| Sham与LPS相比,P< 0.01;L与LPS相比,P< 0.05;aP< 0.05, bP< 0.01。 图 2 rhTPO对LPS诱导TCP小鼠洗涤血小板CD61/CD62p表达的影响 Fig 2 Effect of rhTPO on expression of CD61/CD62p in washed platelets of mice with LPS- induced thrombocytopenia |
|
|
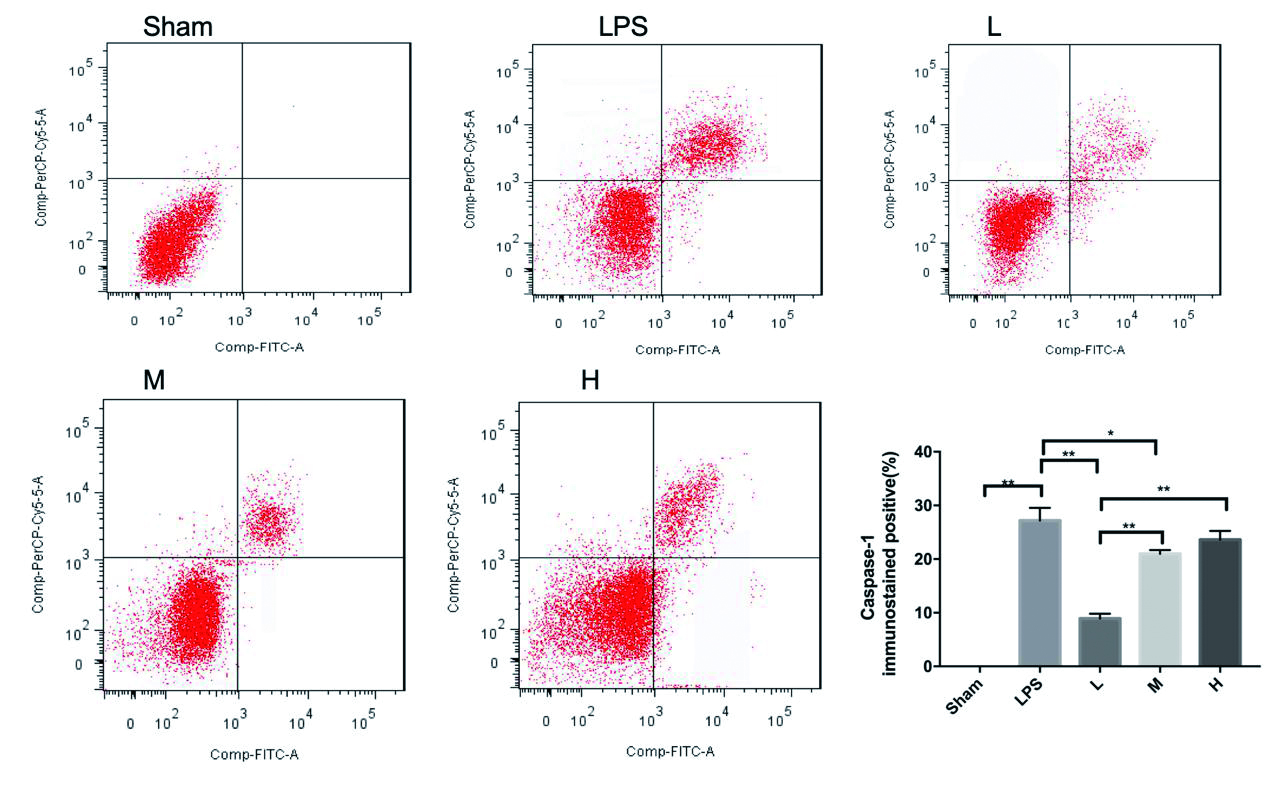
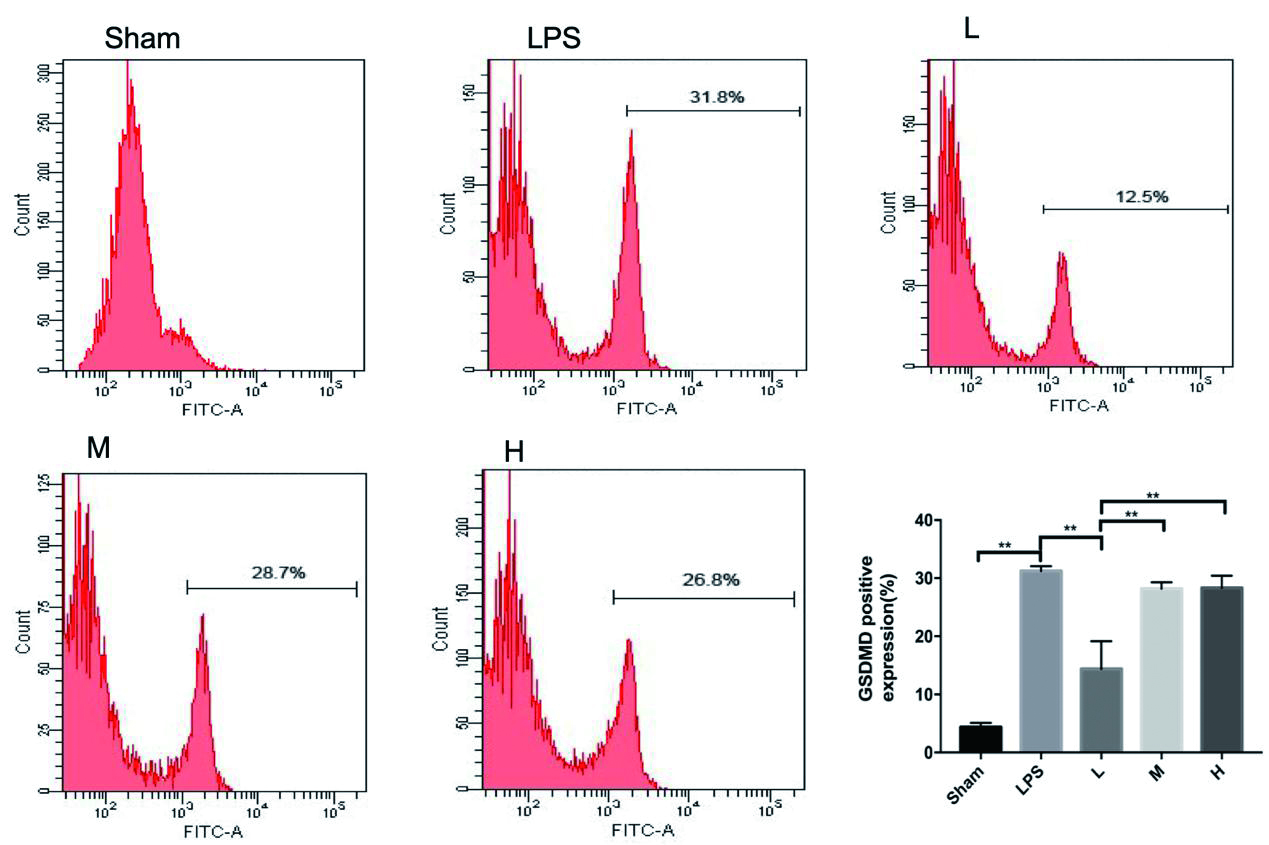
LPS组小鼠洗涤血小板GSDMD蛋白较Sham组明显升高(P< 0. 001),L组较LPS组下降明显(P= 0. 002),M、H组较LPS组变化不明显(P= 0.753,P= 0.827),见图 3。Caspase-1表达LPS组小鼠较Sham组明显升高(P< 0. 01),L组较LPS组下降明显(P< 0. 001),M组较LPS组下降(P= 0. 017),差异均有统计学意义。H组较LPS组变化不明显(P= 0. 158),差异无统计学意义。见图 4。
|
| Sham与LPS比,P< 0.01;L与LPS相比,P< 0.01;M与LPS相比P< 0.05;bP< 0.01,aP< 0.05。 图 3 rhTPO对LPS诱导TCP小鼠Caspase-1蛋白的表达 Fig 3 Expression of Caspase-1 in mice with LPS-induced thrombocytopenia by rhTPO |
|
|
|
| Sham与LPS相比,P< 0.01;L与LPS相比,P< 0.01;bP< 0.01。 图 4 rhTPO对LPS诱导TCP小鼠GSDMD蛋白的表达 Fig 4 Expression of GSDMD in mice with LPS-induced thrombocytopenia by rhTPO |
|
|
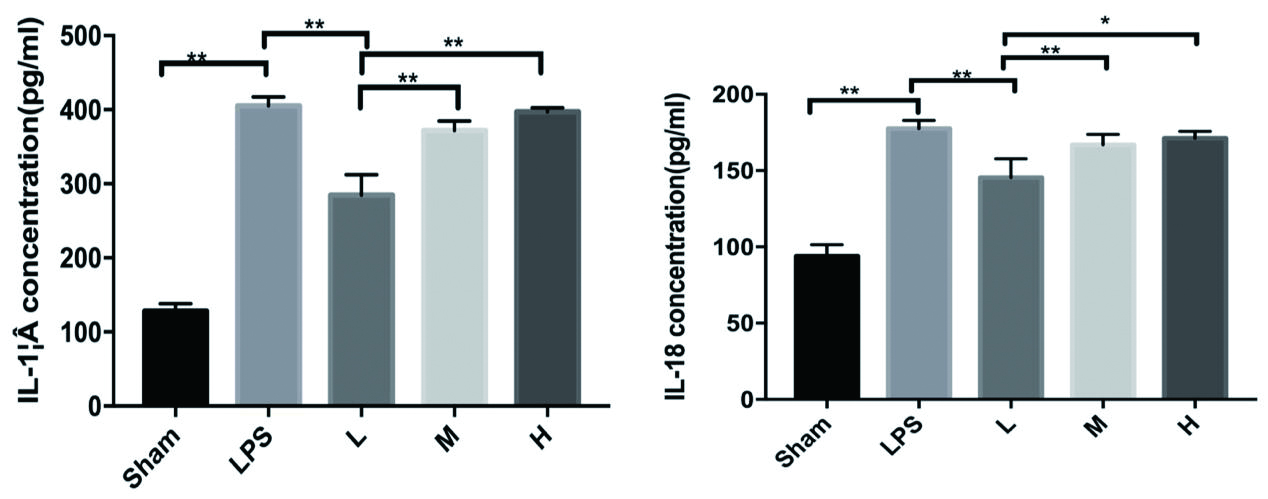
LPS组小鼠富血小板血浆IL-1β、IL-18水平较Sham组明显升高,P< 0. 001,差异有统计学意义。L组较LPS组下降明显(P< 0. 001),M、H组较LPS组变化不明显(P= 0.084,P= 0.983), P均 > 0. 05,差异无统计学意义。见图 5。
|
| LPS与Shame相比,P<0.01;L与LPS相比,P<0.01;aP<0.05, bP<0.01 图 5 rhTPO对LPS诱导TCP小鼠血浆IL-1β、IL-18的表达 Fig 5 Expression of IL-1β and IL-18 in plasma of mice with LPSinduced thrombocytopenia by rhTPO |
|
|
脓毒症是宿主感染后免疫反应失调引起可能危及生命的器官功能障[6],其特征是活化的白细胞、血小板和内皮细胞之间相互作用,以及细胞因子的连续释放[7-8]。作为先天免疫系统的重要成员,血小板是脓毒症的发病机制的关键参与者之一[9-10]。血小板减少症被认为是严重脓毒症或脓毒性休克患者死亡的独立危险因素[11]。经LPS诱导的小鼠血小板减少症模型是TCP基础研究的常用模型之一。本研究表明,小鼠腹腔注射LPS后小鼠血小板计数开始下降,且LPS组小鼠72 h生存率仅为45%,证明LPS注射后诱发小鼠确实出现了血小板减少症,本研究TCP模型构建成功。血小板生成素(thrombopoietin,TPO)是血小板生成过程中重要生长因子,具有刺激巨核细胞增殖和分化的能力,与内源性血小板生成素有相似的升血小板作用。本研究发现,rhTPO治疗组较LPS组小鼠72 h生存率差异有统计学意义,但低剂量rhTPO明显增加了TCP小鼠的血小板计数,这表明rhTPO对LPS诱导的TCP小鼠具有一定的治疗作用。
血小板活化在脓毒症所致TCP中起主要作用,因为它们促进白细胞活化、白细胞与内皮细胞的黏附以及白细胞的跨内皮细胞迁移[1]。内毒素血症模式及脓毒症模式动物均易出现严重的血小板减少症,同时伴随离体血小板活化指数增加[8]。脓毒症和受损内皮细胞的炎症—凝血反应诱导血小板活化,这些活化的血小板可通过与炎症和内皮细胞的相互作用以及其他机制进一步加剧全身炎症反应和凝血障碍[8]。本研究结果显示,第72小时LPS组小鼠洗涤血小板CD61/CD62p表达较Sham组明显升高,血小板活化明显,这与国际上的研究结果是一致的。本研究还发现L组CD61/CD62p表达较LPS组明显下降,这表明rhTPO在上升血小板水平的同时具有抑制血小板的活化的作用。
焦亡(Pyroptosis)是一种由caspase-1介导的细胞程序性细胞死亡形式,通过GSDMD的裂解使细胞膜功能障碍,细胞内容物与促炎细胞因子一起流出,促进活化的IL-1β和IL-18的释放,引起局部或全身炎症反应[12]。通常,焦亡保护多细胞宿主生物免受侵入性致病细菌和微生物感染; 但是,它也导致脓毒症和感染性休克[13]。血小板在炎症期间也释放含有免疫调节蛋白的细胞质颗粒,包括趋化因子,黏附蛋白和凝血因子的α-颗粒等,这些颗粒通常储存在细胞质,并且可以刺激后释放以促进免疫调节[14]。这一释放的过程与细胞焦亡释放炎症因子,并伴随炎症反应的过程相似。本研究发现,LPS诱导的TCP小鼠GSDMD蛋白及Caspase-1水平较Sham组明显升高,而且富血小板血浆中炎症因子IL-1β和IL-18水平也明显升高。这提示了内毒素血症小鼠血小板存在焦亡现象的可能,并且低剂量rhTPO抑制了血小板焦亡的发生,并通过减少炎症因子IL-1β和IL-18的释放的形式,改善机体炎症反应状态。
在本研究中与低剂量rhTPO相比,较高剂量rhTPO应用后血小板计数升高不明显,而且对血小板活化及焦亡没有明显抑制。较高剂量rhTPO对机体没有起到预想的治疗保护作用,可能的原因为TPO在体内除了可以升高血小板水平外,也可以放大其他生物介质的作用,加重器官损伤[11]。有研究表明,血小板是TPO的存储池,且TPO极有可能存在于血小板颗粒中; 在炎症状态下,各种刺激因素使血小板活化并释放TPO,同时促进肝细胞内TPO的合成[15]。因此,内毒素血症和脓毒症患者特别是严重脓毒症和感染性休克的患者其体内TPO处于较高水平。TPO在体内主要通过结合c-mpl受体发挥作用,此结合过程是一种可饱和的过程。外源性高剂量TPO的注入饱和了其与血小板c-mpl受体的结合能力,更有可能加重脏器功能的损伤[14]。本研究结果显示,较高剂量rhTPO升高血小板计数、降低病死率效果不明显,这进一步表明rhTPO的应用可能存在阈值效应,而且最佳rhTPO的药物浓度可能在低中剂量之间。
| [1] | Larkin CM, Santos-Martinez MJ, Ryan T, et al. Sepsis-associated thrombocytopenia[J]. Thromb Res, 2016, 141(2): 11-16. DOI:10.1016/j.thromres.2016.02.022 |
| [2] | 陈登科, 刘照玉, 王玉兰, 等. 重组血小板生成素联合地塞米松治疗原发免疫性血小板减少症的疗效[J]. 中国老年学杂志, 2015, 35(3): 628-630. DOI:10.3969/j.issn.1005-9202.2015.03.025 |
| [3] | Zhang X, Chuai Y, Nie W, et al. Thrombopoietin receptor agonists for prevention and treatment of chemotherapy-induced thrombocytopenia in patients with solid tumours[J]. Cochrane Database Syst Rev, 2017, 11: D12035. DOI:10.1002/14651858.cd012035 |
| [4] | 章渭方, 方君俊, 王国彬, 等. 重组人血小板生成素治疗脓毒症相关血小板减少症患者的临床研究[J]. 中华危重症医学杂志(电子版), 2016(5): 300-308. DOI:10.3877/cma.j.issn.1674-6880.2016.05.004 |
| [5] | Soriano FG, Lorigados CB, Pacher P, et al. Effects of a potent peroxynitrite decomposition catalyst in murine models of endotoxemia and sepsis[J]. Shock, 2011, 35(6): 560-566. DOI:10.1097/SHK.0b013e31820fe5d5 |
| [6] | Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)[J]. JAMA, 2016, 315(8): 801-810. DOI:10.1001/jama.2016.0287 |
| [7] | Granja T, Körner A, Glück C, et al. Targeting CD39 toward activated platelets reduces systemic inflammation and improves survival in sepsis[J]. Crit Care Med, 2019, 47(5): e420-e427. DOI:10.1097/ccm.0000000000003682 |
| [8] | Vardon-Bounes F, Ruiz S, Gratacap MP, et al. Platelets Are Critical Key Players in Sepsis[J]. Int J Mol Sci, 2019, 20(14). DOI:10.3390/ijms20143494 |
| [9] | Dewitte A, Lepreux S, Villeneuve J, et al. Blood platelets and sepsis pathophysiology: A new therapeutic prospect in critical ill patients?[J]. Ann Intensive Care, 2017, 7: 115. DOI:10.1186/s13613-017-0337-7 |
| [10] | Assinger A, Schrottmaier WC, Salzmann M, et al. Platelets in Sepsis: An Update on Experimental Models and Clinical Data[J]. Front Immunol, 2019, 10: 1687. DOI:10.3389/fimmu.2019.01687 |
| [11] | Cuccurullo A, Greco E, Lupia E, et al. Blockade of thrombopoietin reduces organ damage in experimental endotoxemia and polymicrobial sepsis[J]. PLoS One, 2016, 11(3): e151088. DOI:10.1371/journal.pone.0151088 |
| [12] | Ma Y, Jiang J, Gao Y, et al. Research progress of the relationship between pyroptosis and disease[J]. Am J Transl Res, 2018, 10(7): 2213-2219. |
| [13] | Aglietti RA, Dueber EC. Recent insights into the molecular mechanisms underlying pyroptosis and gasdermin family functions[J]. Trends Immunology, 2017, 38(4): 261-271. DOI:10.1016/j.it.2017.01.003 |
| [14] | Greco E, Lupia E, Bosco O, et al. Platelets and multi-organ failure in sepsis[J]. Int J Mol Sci, 2017, 18(10). DOI:10.3390/ijms18102200 |
| [15] | Folman C, Linthorst G, van Mourik J, et al. Platelets release thrombopoietin (tpo) upon activation: another regulatory loop in thrombocytopoiesis?[J]. Thromb Haemost, 2000, 83(6): 923-930. DOI:10.1055/s-0037-1613944 |
 2019, Vol. 28
2019, Vol. 28




