2 宁夏医科大学总医院住院医师规范化培训基地,银川 750004;
3 宁夏医科大学总医院重症医学科,银川 750004
2 Resident Standardized Training Base, General Hospital of Ningxia Medical University, Yinchuan 750004, China;
3 Department of Critical Care Medicine, General Hospital of Ningxia Medical University, Yinchuan 750004, China
TNF-α是急性肺损伤(acute lung injury, ALI)时介导炎症反应的主要因子,参与ALI的发生及发展[1]。肺上皮屏障调控并维持肺内外液体平衡,是ALI的关键病理部位。肺泡上皮细胞和细胞间紧密连接共同维持肺上皮屏障的功能[2]。紧密连接为细胞-细胞间连接复合体,主要由Claudins、Occludin、ZO-1等组成,调控细胞旁通透性[3-5]。本研究通过探究肿瘤坏死因子-α(TNF-α)对肺泡Ⅱ型上皮细胞及其紧密连接蛋白Claudin-4、ZO-1表达的影响,初步探讨TNF-α对肺上皮屏障功能的影响。
1 材料与方法 1.1 体外培养大鼠肺泡Ⅱ型上皮细胞大鼠肺泡Ⅱ型上皮细胞(alveolar epithelial type Ⅱ cells, AEC-Ⅱ)(上海拜力生物有限公司,中国)。将细胞分为正常对照组:用含10%胎牛血清(Gibco公司,美国)的DME F12(HyClone公司,美国)培养液孵育贴壁细胞。TNF-α24 h、48 h、72 h组:分别用等体积的TNF-α10 ng/mL(Pepprotech公司,美国)培养液刺激贴壁细胞24 h、48 h、72 h。
1.2 透射电镜鉴定AEC-Ⅱ并观察细胞超微结构收集对照组及处理结束后的各组细胞,用0.1 mol/L二甲砷酸钠缓冲液洗涤细胞,2%戊二醛重悬细胞,4 ℃放置1 h。1%锇酸浸泡1 h,依次用30%、50%、70%、80%、90%、100%的酒精脱水,环氧丙烷渗透,包埋液包埋,用超薄切片机切成50 nm的切片,3%醋酸枸橼酸银染色。透射电子显微镜(日立H7650,日本)观察细胞超微结构。
1.3 MTT法测定细胞增殖抑制率取对数生长期的细胞,细胞计数,调整细胞密度为1×105个/mL,以5 000个细胞/孔的密度均匀接种于96孔板中,置于37 ℃、含5% CO2的培养箱中孵育24 h,按照实验分组处理细胞,每组设置3个复孔,处理结束后,更换为无血清培养基90 μL/孔,加入20 μL 1×MTT溶液,37 ℃避光孵育4 h,将MTT还原为甲瓒,加入150 μL DMSO溶解甲瓒,水平振荡10 min摇匀,酶标仪在490 nm波长处测定每孔吸光度值(A值)。计算细胞增殖抑制率=(A对照组-A实验组)/A对照组×100%。
1.4 流式双染法测定细胞早期凋亡率取对数生长期的细胞以1×105个/mL接种于六孔板中,置于37℃、5% CO2的培养箱中培养24 h后,按照实验分组处理细胞,每组设2个复孔,处理结束后,按照Annexin V-FITC/PI双染细胞凋亡检测试剂盒(上海贝博生物公司,中国)说明书进行操作,用流式细胞仪检测细胞早期凋亡率。
1.5 免疫荧光法观察紧密连接蛋白ZO-1、Claudin-4的表达及分布将生长状态良好的AEC-Ⅱ细胞以1×105个/mL均匀接种于置有无菌爬片的六孔板内,置于37℃、5% CO2的培养箱中培养24 h,按照实验分组处理细胞,处理结束后用多聚甲醛固定20 min,PBS洗涤细胞,5%BSA室温封闭1 h,Claudin-4抗体(1:50,Invitrogen公司,美国),ZO-1抗体(1:50,Invitrogen公司,美国)4 ℃孵育过夜,洗涤,用FITC标记的二抗(1:50,北京中杉金桥生物公司,中国)室温避光孵育1 h,再洗涤、DAPI染核封片。共聚焦显微镜(Olympus FV1000,Olympus公司,美国)观察并拍照。
1.6 实时荧光定量PCR测定Claudin-4、ZO-1 mRNA水平将生长状态良好的AEC-Ⅱ以适当密度均匀接种于六孔板内,孵育24 h后,按照实验分组处理细胞,处理结束后,提取细胞总RNA(Trizol法),反转录成cDNA,应用LightCycler480 System进行Real-time PCR反应。记录CT值。β-actin作为内参。
1.7 Western blot法测定紧密连接蛋白ZO-1、Claudin-4的表达将细胞均匀接种于六孔板内,孵育24 h后,按照实验分组干预细胞,干预结束后,提取细胞总蛋白,BCA法测定总蛋白浓度,配胶、上样、电泳,转膜(300 mA,1.5 h),5%脱脂牛奶封闭2 h,分别加入Claudin-4抗体(1:500,Invitrogen公司,美国),ZO-1抗体(1:250,Invitrogen公司,美国),β-actin抗体(1:500,北京中杉金桥生物技术有限公司,中国)4℃孵育过夜,洗涤,用辣根过氧化物酶标记的二抗(1:5 000,北京中杉金桥生物公司,中国)室温孵育2 h,洗涤,ECL化学发光显影,曝光,成像。用Image Lab分析软件进行图像的分析处理,并测定各条带的灰度值,用目的条带的灰度值/对应内参的灰度值,反映各组蛋白相对表达的变化。
| 引物名称 | 引物序列 | 扩增产物长度 |
| Claudin-4反向 | 5’- AGAAGTCGCGGATGACGTTGTG-3’ | 296 bp |
| Claudin-4正向 | 5’-CCTGTGGATGAACTGCGTGGTG-3’ | 296 bp |
| ZO-1反向 | 5’-TGCTATTACACGGTCCTC-3’ | 642 bp |
| ZO-1正向 | 5’-TGGTGCTCCTAAACAATC-3’ | 642 bp |
| β-actin反向 | 5’-TTTAATGTCACGCACGATTTC-3’ | 150 bp |
| β-actin正向 | 5’-CCCATCTATGAGGGTTACGC-3’ | 150 bp |
采用SPSS 17.0统计软件进行统计分析。计量资料以均数±标准差(Mean±SD)表示,对数据进行正态性和方差齐性检验,若满足正态性,方差齐,则多组间均数比较采用单因素方差分析,组间两两比较采用SNK-q检验。若不符合正态性,方差不齐,则用秩转换的非参数检验。以P < 0.05为差异有统计学意义。
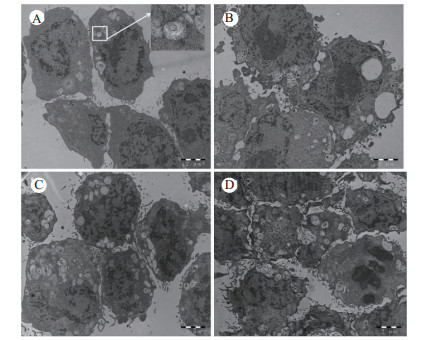
2 结果 2.1 TNF-α对AEC-Ⅱ超微结构的影响透射电镜鉴定AEC-Ⅱ,观察结果如图 1所示。镜下可见对照组AEC-Ⅱ细胞表面有数量不等、长短不一的微绒毛,胞质均匀,可见线粒体、高尔基体、内质网等细胞器,并可见典型的板层小体结构,数量不等,大小不一,成熟阶段不同,围绕细胞核散在分布。细胞核染色质均匀。确定本研究所用细胞为肺泡Ⅱ型上皮细胞。TNF-α刺激细胞24 h即可见细胞的形态发生改变,细胞内板层小体逐渐空泡化,线粒体及内质网水肿,随着作用时间的延长,细胞形态扭曲更明显,板层小体排空现象更为严重,线粒体水肿明显加重,嵴逐渐溶解、断裂,直至消失。细胞核固缩,核溶解,视野中凋亡细胞比例增加。
|
| A:正常对照组; B: TNF-α 24 h组; C:TNF-α 48 h组; D:TNF-α 72 h组 图 1 大鼠AEC-Ⅱ超微结构及TNF-α刺激后变化(×10 000,醋酸枸橼酸银染色) Fig 1 Ultrastructure of rat AEC-Ⅱ and changes after TNF-α intervention (×10 000, silver citrate acetate staining) |
|
|
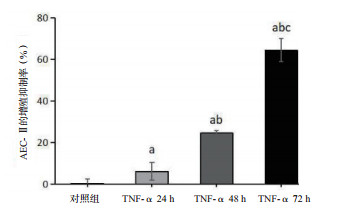
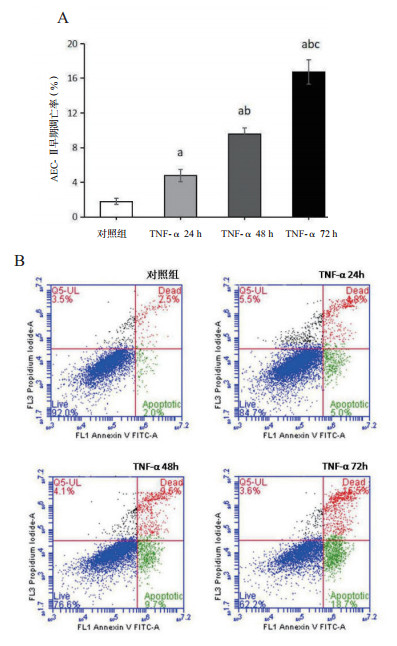
TNF-α刺激细胞24 h时,细胞的增殖抑制率(6.15±4.33)%明显高于对照组(0.00±2.17)%(P < 0.05); 随着刺激时间的延长,细胞的增殖抑制率逐渐升高[48 h:(24.55±1.10)%; 72 h:(64.40±5.59)%],各组间差异均有统计学意义(均P < 0.01)。TNF-α 24 h组细胞的早期凋亡率(4.77±0.72)%较对照组(1.80±0.36)%明显升高(P < 0.01),TNF-α刺激48 h、72 h时,细胞的早期凋亡率[(9.57±0.71)%; (16.73±1.38)%]继续增加(均P < 0.01); 从流式细胞仪分析图(图 3B)中也可见; 细胞的早期凋亡率(右下象限)在TNF-α刺激后逐渐升高,说明TNF-α抑制AEC-Ⅱ细胞的增殖,诱导其凋亡,具有时间依赖效应。见图 2~3。
|
| 与对照组比较, aP < 0.05;与TNF-α 24 h比较, bP < 0.05;与TNF-α 48 h比较,cP < 0.05 图 2 AEC-Ⅱ增殖抑制率的变化 Fig 2 Changes of proliferation inhibition rate of AEC-Ⅱ cells |
|
|
|
| (A)与对照组比较, aP < 0.05;与TNF-α 24 h比较, bP < 0.05;与TNF-α 48 h比较,cP < 0.05;(B)十字线把图分为四个象限,左上象限代表坏死细胞比例,左下象限表示正常细胞比例,右上象限为晚期凋亡细胞比例,右下象限为早期凋亡细胞比例 图 3 AEC-Ⅱ早期凋亡率的变化 Fig 3 Changes of early apoptotic rate of AEC-Ⅱcells |
|
|
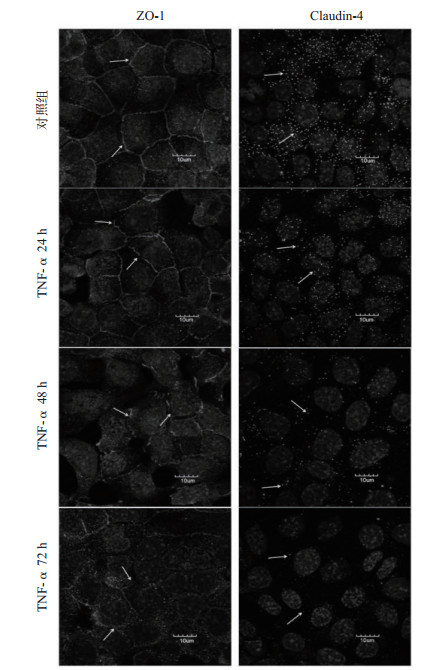
对照组ZO-1沿着细胞膜的内表面分布,镜下呈连续的线状带分布在细胞的边缘及细胞连接部位,在细胞间连接成网。TNF-α作用24 h时,ZO-1蛋白的荧光强度与对照组相比稍减弱,但线性结构基本完整,仅有少部分发生断裂。48 h时,ZO-1线性结构断裂明显,镜下可见断续的线状片段,少见完整的网状结构,ZO-1向胞质内移位。作用72 h时,ZO-1的荧光强度显著降低,几乎看不到线性结构,呈现散在分布的荧光点。对照组Claudin-4在细胞膜表面呈散点状荧光分布,TNF-α作用24 h时,Claudin-4荧光点强度较对照组减弱,数量较对照组明显减少; 继续作用至48 h时,Claudin-4的荧光强度继续减弱,荧光密度继续降低; 到72 h时,视野中仅可见少量极其微弱的荧光点,有的视野中甚至无荧光显像,见图 4。
|
| 图 4 紧密连接蛋白ZO-1、Claudin-4在大鼠AEC-Ⅱ的表达分布变化(箭头示紧密连接蛋白ZO-1、Claudin-4) Fig 4 Changes in the expression and distribution of tight junction proteins ZO-1 and Claudin-4 in rat AEC-Ⅱcells (the arrow indicates ZO-1 or Claudin-4) |
|
|
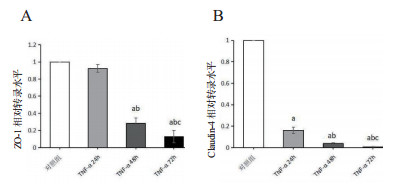
TNF-α24 h组ZO-1转录水平(0.92±0.05)与对照组比较无明显变化(P > 0.05),TNF-α48 h组的ZO-1 mRNA水平(0.28±0.06)明显下降(P < 0.01),TNF-α72 h组的ZO-1转录水平继续降低(0.13±0.07)。而Claudin-4的mRNA水平在TNF-α刺激细胞24 h即出现显著降低(0.16±0.03),与对照组比较,差异有统计学意义(P < 0.01)。TNF-α继续作用至48 h、72 h,Claudin-4 mRNA表达水平继续降低(0.04±0.01;0.01±0.00),各组间差异均有统计学意义(P < 0.05),见图 5。
|
| 与对照组比较, aP < 0.05;与TNF-α 24 h比较, bP < 0.05;与TNF-α 48 h比较,cP < 0.05 图 5 大鼠AEC-Ⅱ的ZO-1(A)、Claudin-4(B)mRNA水平的变化 Fig 5 The changes of ZO-1 (A) and Claudin-4 (B) mRNA levels in rat AEC-Ⅱcells |
|
|
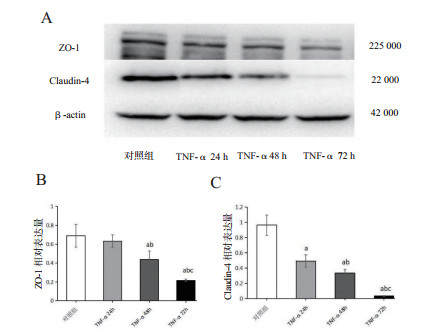
TNF-α 24 h组ZO-1蛋白的表达(0.63±0.07)较对照组(0.69±0.12)降低(P > 0.05),48 h组ZO-1的表达(0.44±0.09)较24 h组减低(P < 0.05),72 h组ZO-1蛋白表达(0.2±0.01)较48 h组显著降低(P < 0.05)。TNF-α各组细胞紧密连接蛋白Claudin-4的表达(24 h:0.49±0.08;48 h:0.34±0.05;72 h:0.04±0.01)均较对照组(0.96±0.13)降低(P < 0.01),且随着作用时间的延长,蛋白表达逐渐减少,各组间比较差异均有统计学意义(P < 0.05),见图 6。
|
| (A)ZO-1、Claudin-4、β-actin蛋白条带; ZO-1(B)、Claudin-4(C)表达的变化; 与对照组比较, aP < 0.05;与TNF-α 24 h比较, bP < 0.05;与TNF-α 48 h比较,cP < 0.05 图 6 大鼠AEC-Ⅱ紧密连接蛋白ZO-1、Claudin-4蛋白表达的变化 Fig 6 The changes of ZO-1 and Claudin-4 proteins expression in rat AEC-Ⅱcells |
|
|
肺屏障由肺上皮屏障及肺微血管内皮屏障共同组成,肺上皮屏障较肺微血管屏障更为紧密[6],肺泡上皮对水溶性溶质的有效孔隙半径为0.9 nm,远小于微血管内皮细胞的7.5 nm,是诱导肺水肿发生的关键屏障结构[7]。在各种原因导致的ALI中,肺泡上皮屏障功能受损也是ALI的重要病理生理变化,而残存的上皮屏障功能与患者病死率呈负相关[8]。
TNF-α在细胞死亡及免疫细胞的募集和激活中至关重要,被认为是严重疾病的全身进展和组织损伤的标志性介质之一[9]。本研究发现TNF-α作用于大鼠AEC-Ⅱ后,细胞形态发生扭曲变形,细胞结构明显损伤,随着作用时间的延长,细胞内板层小体逐渐排空,线粒体水肿,内质网扩张,还可见大量溶酶体样结构。因此,TNF-α可直接损伤大鼠肺泡Ⅱ型上皮细胞。
本研究检测了TNF-α对大鼠AEC-Ⅱ增殖的影响,研究显示,TNF-α抑制AEC-Ⅱ的增殖,而AEC-Ⅱ为修复肺损伤、分泌表面活性物质的主要细胞,抑制AEC-Ⅱ的增殖将阻碍损伤肺组织的自我修复。研究显示,急性肺损伤时伴有肺泡Ⅱ型上皮细胞的凋亡[10-11]。在炎症反应时,TNF-α过表达通过活化Caspase-3,与肿瘤坏死因子受体1(TNFR1)结合等多种途径诱导细胞凋亡[12-13]。本研究结果显示TNF-α各组细胞早期凋亡率均较对照组升高,且随着作用时间的延长,凋亡率逐渐升高。在电镜下也观察到TNF-α组视野内部分细胞发生了核固缩,溶解,碎裂的现象,与流式细胞仪结果一致。说明TNF-α抑制大鼠AEC-Ⅱ的增殖,并诱导其凋亡,从而破坏肺上皮屏障的连续性,阻碍肺组织的自我修复,是导致上皮屏障通透性增加的重要原因。
调控肺上皮屏障功能更为关键的结构为肺上皮细胞间的紧密连接[14-15]。紧密连接是一种细胞间连接复合体,高度定位于细胞与细胞连接部位[16]。本研究通过免疫荧光染色,共聚焦显微镜下观察,紧密连接结构蛋白ZO-1在大鼠AEC-Ⅱ高表达,沿细胞膜呈线状分布,在相邻细胞间交织成网,形成横向等渗膜。跨膜蛋白Claudin-4在细胞膜表面呈散点状分布,与ZO-1共同调节细胞旁通透性。
本研究用TNF-α刺激细胞后,共聚焦显微镜下观察到细胞ZO-1的线状荧光出现断裂,随着作用时间的延长,部分线性结构消失,向胞质内移位。到72 h时,ZO-1线状结构基本消失,只呈现散在的荧光点。而ZO-1可以调节Claudin蛋白与紧密连接的结合,从而调节细胞旁离子交换[14]。降低ZO-1的表达,可以使Claudin蛋白易于从紧密连接结构中脱落,从而使细胞间的紧密连接松弛[17]。也有研究发现,TNF-α可以重新分布紧密连接蛋白,使紧密连接结构移位,从而影响细胞旁通透性[18]。本研究也观察到TNF-α各组细胞Claudin-4的荧光强度较对照组明显降低,随着作用时间的延长,荧光点密度逐渐降低。说明TNF-α破坏ZO-1及Claudin-4在AEC-Ⅱ细胞的结构和改变紧密连接蛋白在细胞的分布,使紧密连接松弛。
肺上皮细胞主要表达Claudin-4、Claudin-18和Claudin-3,其中Claudin-4在肺泡液体清除中发挥关键作用[19]。本课题组在前期研究中已发现急性肺损伤大鼠肺组织Claudin-5及ZO-1的表达下调,肺湿/干比值升高[20];上调肺微血管内皮细胞Claudin-5、ZO-1的表达,保护肺损伤[21]。说明Claudins及ZO-1表达的变化与肺损伤密切相关。本研究检测了TNF-α作用后的大鼠AEC-Ⅱ的Claudin-4、ZO-1的mRNA水平,发现细胞Claudin-4及ZO-1 mRNA的转录较对照组降低,在蛋白表达的实验中,同样发现TNF-α组Claudin-4及ZO-1的表达水平较对照组降低。这也同时印证了免疫荧光实验中观察到的TNF-α各组Claudin-4的荧光强度减低,荧光强度减弱; ZO-1的线性荧光强度减弱的结果。Yang等[22]的研究也发现下调紧密连接蛋白Claudin-4、ZO-1、Occludin的表达可以降低肺泡Ⅱ型上皮细胞旁通透性,影响肺上皮屏障功能。因此,本研究中TNF-α降低AEC-Ⅱ的Claudin-4及ZO-1的转录,下调其表达,影响肺屏障。然而,关于TNF-α诱导AEC-Ⅱ细胞凋亡与下调细胞紧密连接蛋白表达是否存在直接相关性,目前尚未被证实,笔者将在今后的研究中继续深入探讨,进一步明确TNF-α损伤肺上皮屏障的更深一层机制。
综上所述,TNF-α损伤大鼠肺泡Ⅱ型上皮细胞,诱导其凋亡,破坏肺上皮屏障的连续性。同时,下调Claudin-4、ZO-1的转录及表达,改变其定位,破坏细胞间紧密连接结构,损伤肺上皮屏障。
| [1] | An S, Hishikawa Y, Jie L, et al. Lung injury after ischemia-reperfusion of small intestine in rats involves apoptosis of typeⅡalveolar epithelial cells mediated by TNF-α and activation of Bid pathway[J]. Apoptosis, 2007, 12(11): 1989-2001. DOI:10.1007/s10495-007-0125-1 |
| [2] | Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury[J]. Annu Rev Physiol, 2013, 75: 593-615. DOI:10.1146/annurev-physiol-030212-183756 |
| [3] | Overgaard CE, Mitchell LA, Koval M. Roles for claudins in alveolar epithelial barrier function[J]. Ann N Y Acad Sci, 2012, 1257(1): 167-174. DOI:10.1111/j.1749-6632.2012.06545.x |
| [4] | Xin C, Yan Z, Jing L, et al. Claudin-4 is required for modulation of paracellular permeability by muscarinic acetylcholine receptor in epithelial cells[J]. J Cell Sci, 2015, 128(12): 2271-2286. DOI:10.1242/jcs.165878 |
| [5] | Fanning AS, Itallie CMV, Anderson JM. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia[J]. Mol Bio Cell, 2012, 23(4): 577-590. DOI:10.1091/mbc.E11-09-0791 |
| [6] | Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial[J]. JAMA, 2008, 299(6): 646-655. DOI:10.1001/jama.299.6.646 |
| [7] | 毕继蕊, 杨进, 汪影, 等. 丙咪嗪对小鼠急性肺损伤肺泡上皮屏障功能保护作用的研究[J]. 中华急诊医学杂志, 2017, 26(6): 638-643. DOI:10.3760/cma.j.issn.1671-0282.2017.06.008 |
| [8] | Frank JA. Claudins and alveolar epithelial barrier function in the lung[J]. Ann N Y Acad Sci, 2012, 1257(1): 175-183. DOI:10.1111/j.1749-6632.2012.06533.x |
| [9] | Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution[J]. FASEB J, 2006, 20(10): 1589-1598. DOI:10.1096/fj.05-5603rev |
| [10] | Tesfaigzi J, Wood MB, Johnson NF, et al. Apoptosis is a pathway responsible for the resolution of endotoxin-induced alveolar type Ⅱ cell hyperplasia in the rat[J]. Int J Exper Pathol, 1998, 79(5): 303-311. DOI:10.1046/j.1365-2613.1998.720402.x |
| [11] | Husari AW, Dbaibo GS, Bitar H, et al. Apoptosis and the activity of ceramide, Bax and Bcl-2 in the lungs of neonatal rats exposed to limited and prolonged hyperoxia[J]. Respir Res, 2006, 7: 100. DOI:10.1186/1465-9921-7-100 |
| [12] | 钟明, 魏玲玲, 杨显富. 外源性及内源性细胞凋亡机制研究进展[J]. 实用医院临床杂志, 2014, 11(2): 170-174. |
| [13] | Guthmann F, Wissel H, Schachtrup C, et al. Inhibition of TNFalpha in vivo prevents hyperoxia-mediated activation of caspase 3 in type Ⅱ cells[J]. Respir Res, 2005, 6: 1-16. DOI:10.1186/1465-9921-6-10 |
| [14] | Ohta H, Chiba S, Ebina M, et al. Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis[J]. Am J Physiol Lung Cell Mol Physiol, 2012, 302(2): 193-205. DOI:10.1152/ajplung.00349.2010 |
| [15] | Wittekindt OH. Tight junctions in pulmonary epithelia during lung inflammation[J]. Pflugers Archiv, 2017, 469(1): 135-147. DOI:10.1007/s00424-016-1917-3 |
| [16] | Koval M. Claudin heterogeneity and control of lung tight junctions[J]. Ann Rev Physiol, 2013, 75(1): 551-567. DOI:10.1146/annurev-physiol-030212-183809 |
| [17] | Soini Y. Claudins in lung diseases[J]. Respir Res, 2011, 12: 1-11. DOI:10.1186/1465-9921-12-70 |
| [18] | 王晓红, 刘芳, 马希刚, 等. 一氧化碳释放分子2对休克大鼠肠黏膜上皮屏障的保护作用[J]. 中华急诊医学杂志, 2019, 28(1): 50-55. DOI:10.3760/cma.j.issn.1671-0282.201901.010 |
| [19] | Wray C, Mao Y, Pan J, et al. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury[J]. Am J Physiol Lung Cell Mol Physiol, 2009, 297(2): 219-227. DOI:10.1152/ajplung.00043.2009 |
| [20] | Shao P, Zhu JY, Ding H, et al. Tripterygium hypoglaucum (Levl.) Hutch attenuates oleic acid-induced acute lung injury in rats through up-regulating claudin-5 and ZO-1 expression[J]. Int J Clin Exp Med, 2018, 11(7): 6634-6647. |
| [21] | Zhou WJ, Shi GC, Bai JJ, et al. Colquhounia root tablet protects rat pulmonary microvascular endothelial cells against tnf-α-induced injury by upregulating the expression of tight junction proteins Claudin-5 and ZO-1[J]. Evid Based Complement Alternat Med, 2018, 2018: 1024634. DOI:10.1155/2018/1024634 |
| [22] | Yang J, Wang Y, Liu H, et al. C2-ceramide influences alveolar epithelial barrier function by downregulating Zo-1, occludin and claudin-4 expression[J]. Toxicol Mech Methods, 2017, 27(4): 293-297. DOI:10.1080/15376516.2017.1278812 |
 2019, Vol. 28
2019, Vol. 28




