百草枯(paraquat,PQ)作为一种非选择性除草剂,在全世界范围应用广泛,但因剧烈的毒性,其应用也一直充满争议。百草枯中毒以急性肺损伤和慢性肺纤维化为主要特征。大麻素受体激动剂能减轻次氯酸所诱发的皮肤硬化,Christoph等[1]的研究发现体外应用大麻素受体激动剂WIN55212-2可以减轻慢性胰腺炎的炎症活动和纤维化。基于以上大麻素对皮肤、胰腺等脏器的纤维化具有一定的干预作用,笔者推测大麻素类似物WIN55212-2对肺纤维化可能有一定治疗效果,并以实验加以验证。为了研究WIN55212-2对PQ中毒C57BL/6小鼠的干预作用,笔者所在团队进行了多次预实验,并在查阅相关文献的基础上,选定1 mg和2 mg作为实验干预剂量。
1 材料与方法 1.1 实验材料与药物配制采用健康成年C57BL/6雄性小鼠35只,8周龄大,体质量25~30 g,由上海交通大学医学院附属仁济医院(东院)动物中心提供,所有实验动物在饲养及实验过程中均符合上海交通大学医学院附属仁济医院中心实验室动物伦理要求。WIN55212-2由Engo公司提供,因WIN55212-2为脂溶性药物,需使用Tween 80助溶(10%,中国Biosharp公司)。
1.2 实验动物分组及模型建立实验所用健康成年C57BL/6雄性小鼠35只,随机数字法分为四组。PQ组:PQ中毒组(n=10),WIN 1 mg组:PQ+WIN 1 mg干预组(n=10),WIN 2 mg组:PQ+WIN 2 mg干预组(n=10),对照组(n=5),各组间小鼠体质量差异无统计学意义(P=0.142)。PQ组、WIN 1 mg组、WIN 2 mg组小鼠分别在第1天和第3天按20 mg/kg腹腔注射PQ,建立PQ中毒模型,对照组予注射等剂量的生理盐水[2]。WIN 1 mg组和WIN 2 mg组分别于PQ染毒前1 h按1 mg/kg和2 mg/kg的浓度经腹腔注射WIN55212-2(含10% Tween 80助溶剂),PQ组和对照组注射等容量的生理盐水和Tween 80。从第2周开始,每周注射两次干预药物。各实验组每天分别记录小鼠的一般状况,急性期(14 d)PQ组、WIN 1 mg组、WIN 2 mg组各随机处死5只小鼠,留取血浆、BALF、肺组织标本,对照组在急性期处死3只。剩余小鼠全部于第28天处死,留取上述标本,肺组织行HE染色、Masson染色及免疫组化分析。
1.3 观察结局及取材实验第14天和第28天采用摘除小鼠眼球法取血,并处死实验动物,摘取肺脏,光镜下观察各组小鼠急性期及慢性期肺部形态学变化,酶联免疫吸附法(ELISA)测定小鼠急性期血浆和BALF中TNF-α、IL-6含量变化,免疫组化分析慢性期血浆TNF-α及TGF-β的含量变化。
1.4 统计学方法利用SPSS 22.0进行统计学分析。计量资料采用均数±标准差(x±s)表示, 行正态分布及方差齐性检验,多组间比较采用单因素方差分析,组间两两比较采用Bonferroni法。以P < 0.05为差异有统计学意义。
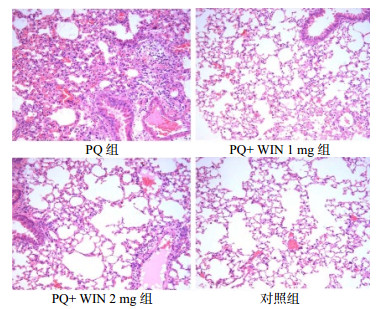
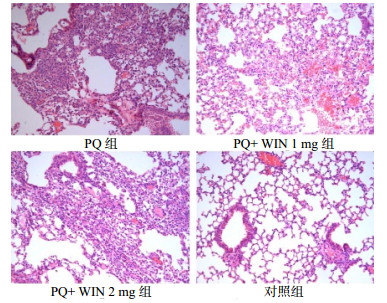
2 结果 2.1 急性期、慢性期肺组织HE染色急性期:PQ组肺淤血严重,灶性出血,肺组织正常结构消失,大量炎细胞(以中性粒细胞为主)浸润,WIN 1 mg组急性炎症表现较PQ组减轻,少量炎症细胞(以中性粒细胞和单核细胞为主)浸润,WIN 2 mg组也表现为急性炎症,肺泡充血明显,对照组为正常肺组织,见图 1。慢性期:PQ组小鼠可见明显慢性炎症伴纤维化,WIN 1 mg组纤维化表现较PQ组减轻,WIN 2 mg组小鼠慢性纤维化表现,纤维化程度略重于WIN 1 mg组,对照组呈正常肺组织形态,见图 2。
|
| 图 1 急性期各组肺组织切片(HE×200) Figure 1 Lung tissue of each group in the acute phase(HE×200) |
|
|
|
| 图 2 慢性期各组肺组织切片(HE×200) Figure 2 Lung tissue of each group in the chronic phase (HE×200) |
|
|
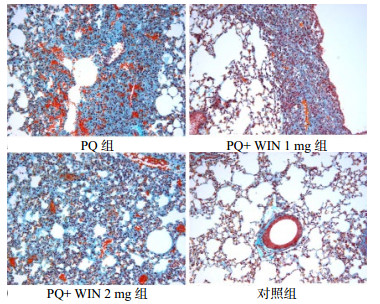
28 d慢性期取各组小鼠肺组织行Masson染色。PQ组可见肺泡组织破坏伴大量绿色胶原沉积;WIN 1 mg组散在浅绿色胶原组织,纤维化程度较PQ组轻,WIN 2 mg组表现为散布深绿色胶原。对照组肺泡结构清晰,无胶原沉积,见图 3。
|
| 图 3 慢性期各组肺胶原沉积(Masson×200) Figure 3 Collagen deposition in lung of each group in the chronic phase (Masson×200) |
|
|
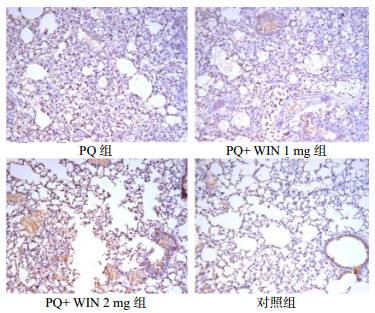
28 d PQ组小鼠肺泡结构破坏,TGF-β大量表达;WIN 1 mg组可见局灶性肺纤维化,肺泡上皮细胞内有TGF-β表达;WIN 2 mg组小鼠可见支气管上皮和间质有散在表达。对照组的肺组织形态相对正常,部分肺泡上皮细胞内有少量表达,见图 4。
|
| 图 4 慢性期各组TGF-β表达(免疫组化×200) Figure 4 TGF-β expression in lung tissue of each group in chornic phase(Immunohistochemistry×200) |
|
|
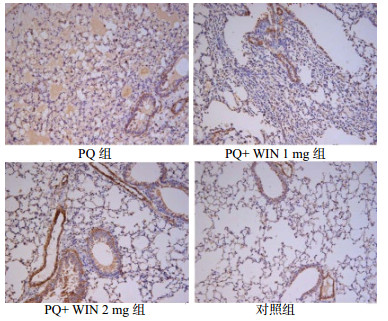
PQ组可见支气管平滑肌、肺泡间质内有阳性染色,WIN 1 mg组肺泡结构相对完整,肺泡间质内有少量表达,WIN 2 mg组在肺泡间质和支气管上皮也有散在表达。对照组小鼠小血管和支气管平滑肌细胞处有α-SMA表达,见图 5。
|
| 图 5 慢性期各组α-SMA表达(免疫组化×200) Figure 5 α-SMA expression in lung tissue of each group in chornic phase(Immunohistochemistry×200) |
|
|
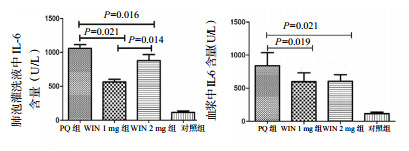
① IL-6 (肺泡灌洗液):PQ组、WIN 1 mg组、WIN 2 mg组、对照组BALF中IL-6水平(U/L)分别为1 024.77±124.74、620.48±99.76、823.29±157.88、180.42±20.22;予WIN55212-2干预后,WIN 1 mg组和WIN 2 mg组IL-6水平均较PQ组下降,且差异有统计学意义(P=0.021,P=0.016)。但两干预组间差异无统计学意义(P=0.114),见图 6。
|
| 图 6 急性期肺泡灌洗液和血浆中IL-6含量 Figure 6 Concentrations of IL-6 in BALF and plasma in the acute phase |
|
|
② IL-6 (血浆):PQ组,WIN 1 mg组,WIN 2 mg组,对照组血浆IL-6水平(U/L)分别为861.34±97.33, 881.54±19.21, 806.91±32.08, 141.12±4.87;予WIN 55212-2干预后,1 mg组和2 mg组IL-6水平均较PQ组下降,且差异有统计学意义(P=0.031,P=0.024),但1 mg组和2 mg组间比较差异无统计学意义(P=0.355),见图 6。
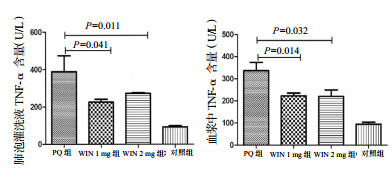
③ TNF-α(肺泡灌洗液):PQ组、WIN 1 mg组、WIN 2 mg组,对照组BALF中IL-6水平(U/L)分别为389.30±85.38、225.71±15.53、272.7±3.51、92.00±7.211;予WIN55212-2干预后,WIN 1 mg组和WIN 2 mg组TNF-α水平均较PQ组下降,且差异统计学意义(P=0.011,P=0.041),但WIN 1 mg组和WIN 2 mg组间比较差异无统计学意义(P=0.460),见图 7。
|
| 图 7 急性期肺泡灌洗液和血浆中TNF-α水平 Figure 7 Concentrations of TNF-αin BALF and plasma in the acute phase |
|
|
④ TNF-α(血浆):PQ组、WIN 1 mg组、WIN 2 mg组、对照组血浆中TNF-α水平(U/L)分别为337.03±37.64、233.33±12.22、220.70±28.87、94.33±9.07;予WIN55212-2干预后,WIN 1 mg组和WIN 2 mg组TNF-α水平均较PQ组下降,且差异有统计学意义(P=0.032,P=0.014),但WIN 1 mg组和WIN 2 mg组间比较差异无统计学意义(P=0.080),见图 7。
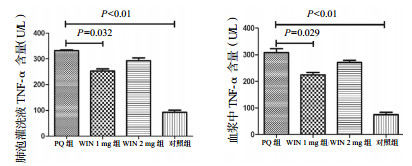
2.4.2 慢性纤维化期肺泡灌洗液及血浆中TNF-α和TGF-β的含量变化① TNF-α(肺泡灌洗液):PQ组、WIN 1 mg组、WIN 2 mg组、对照组BALF中TNF-α水平(U/L)分别为321.64±50.54、260.23±48.19、278.89±29.40、89.76±10.87。WIN 1 mg组水平显著低于PQ组(P=0.032),WIN 2 mg组与PQ组差异无统计学意义(P=0.150),见图 8。
|
| 图 8 慢性期BALF和血浆中TNF-α水平 Figure 8 Concentrations of TNF-αin BALF and plasmain the chronic phase |
|
|
② TNF-α(血浆):PQ组、WIN 1 mg组、WIN 2 mg组、对照组血浆中TNF-α水平(U/L)分别为308.23±19.14、216.32±10.72、267.78±10.24、90.16±14.63。组间比较,WIN 1 mg组水平显著低于PQ组(P=0.029),WIN 2 mg组与PQ组差异无统计学意义(P=0.332),见图 8。
③ TGF-β(肺泡灌洗液):PQ组、WIN 1 mg组、WIN 2 mg组、对照组BALF中TGF-β水平(U/L)分别为424.04±6.14、326.27±21.25、303.93±10.59、118.80±14.41。组间比较,WIN 1 mg组水平显著低于PQ组(P=0.026),但WIN 1 mg组与WIN 2 mg组差异无统计学意义(P=0.349),见图 9。
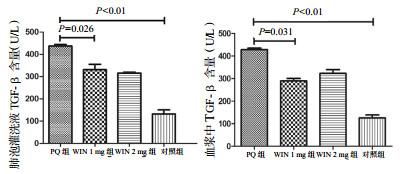
|
| 图 9 慢性期BALF和血浆中TGF-β含量 Figure 9 Concentrations of TGF-βin BALF and plasma in the chronic phase |
|
|
④ TGF-β(血浆):PQ组、WIN 1 mg组、WIN 2 mg组、对照组BALF中TGF-β水平(U/L)分别为414.66±5.44、287.53±10.54、323.91±11.93、116.22±12.11。组间比较,WIN 1 mg组水平显著低于PQ组(P=0.031),但WIN 1 mg组与WIN 2 mg组差异无统计学意义(P=0.057),见图 9。
3 讨论PQ中毒后,约50%分布集中于肺组织,其对肺脏的损伤可以分为急性肺损伤期和慢性肺纤维化期。本实验通过建立PQ中毒小鼠模型,大体及镜下观察了肺组织损伤的急性期和慢性期病理变化,并检测了不同时期相应的炎症因子指标变化。实验中选择WIN55212-2 1 mg/kg和2 mg/kg作为干预剂量,是前期笔者进行了大量的预实验的基础上确定的,预实验发现3 mg/kg、5 mg/kg时剂量偏大,在注射0.5 mg/kg,小鼠没有出现反常活动,中毒后与PQ组表现无异,提示药物作用不够。1 mg/kg和2 mg/kg情况下对小鼠影响较小,干预后较PQ组小鼠有改善,提示1~2 mg/kg可能是合适剂量。有文献报道,WIN55212-2可以诱导低体温,改善心肺复苏术后动物的心脑功能,但是本实验中所用剂量仅为1 mg/kg,一次性腹腔注射,远低于其诱导低温的浓度和剂量,其低体温效应可以忽略[3]。
本研究发现,在急性期PQ中毒小鼠肺组织HE染色呈现广泛而且严重的急性炎症改变,大量炎症细胞浸润,广泛组织破坏,但予WIN55212-2干预后,其炎症状况改善,尤以1 mg/kg时较为明显。虽然急性期相关的炎症指标检测,IL-6和TNF-α与PQ组相比差异无统计学意义,但可以看出用药干预后IL-6和TNF-α有下降趋势。
在慢性期,本研究测定了小鼠血浆和BALF中TNF-α和TGF-β水平,发现在慢性期血浆和BALF中,TNF-α和TGF-β水平较PQ组均明显下降(P < 0.05)。之前相关研究发现TNF-α是PQ中毒后极为重要的细胞因子,在博来霉素引起的纤维化中起重要作用[4]。转化生长因子β(TGF-β)在肺纤维化发展中亦发挥着至关重要的作用[5-6],研究表明其通过增加胶原基因转录来促进肺纤维化[7-8],实验中予WIN55212-2干预后,干预组Masson染色纤维化程度较PQ组改善,提示可能是通过降低TNF-α、TGF-β等炎性因子的表达而发挥作用。
另外,本研究还发现,WIN55212-2干预后慢性期肺组织α-SMA表达减少。有研究发现在PQ所致的肺组织纤维化病程中,肌成纤维细胞过度增殖[9],特征性表达α-SMA,可以作为反映肺纤维化程度的指标[10-11],干预后其表达减少也提示WIN55212-2对慢性期纤维化治疗有一定效果。
大麻素可能通过多条信号通路发挥作用。第一个被发现的大麻素受体是CB1(cannabinoid receptor1),后来又从免疫细胞中发现了CB2(cannabinoid receptor2)[12-13]。CB2分布在免疫细胞上,包括B、T细胞、NK细胞、巨噬细胞。大麻素表现出多效性免疫调节作用,如抑制T细胞增生、抑制抗体产生、促进B细胞生长,抑制细胞因子产生,启动炎症细胞因子向抗炎症细胞因子反应的转换[14]。其发挥免疫抑制作用的途径有:(1)通过抑制cAMP-PKA途径,减少cAMP反应基因的表达;(2)通过IκB-α的磷酸化增加NF-κB所调控的凋亡基因的表达;(3)通过激活P21waf-1/cip-1和诱导G0/G1相,干预细胞周期;(4)通过PPARγ受体,抑制NF-AT的活性[14-15]。大麻素可以干预免疫过程的各个关键点和过程来发挥免疫调节作用。
本实验观察到WIN55212-2可以通过免疫调节和抗炎症反应,降低PQ中毒小鼠肺的细胞因子释放,从而有助于改善肺损伤和肺纤维化,可能临床治疗和干预肺纤维化提供了一条新思路。但是本实验也存在一定的局限,比如实验动物样本量偏少,WIN55212-2的其他药理作用尚未完全明确。因此WIN55212-2对PQ中毒所致的肺纤维的保护作用的具体机制,还需要进一步研究。
| [1] | Michalski CW, Maier M, Erkan M, et al. Cannabinoids reduce markers of inflammation and fibrosis in pancreatic stellate cells[J]. Plos One, 2008, 3(2): e1701. DOI:10.1371/journal.pone.0001701 |
| [2] | 陈丽, 钱洁, 叶延, 等. 百草枯反复小剂量腹腔给药诱导小鼠肺纤维化模型[J]. 中华急诊医学杂志, 2011, 20(12): 1285-1289. DOI:10.3760/cma.j.issn.1671-0282.2011.12.016 |
| [3] | Sun S, Park J. Cannabinoid 1(CB1)receptor mediates WIN55212-2 induced hypothermia and improved survivalin a rat post-cardiac arrest model[J]. Resuscitation, 2012, 83(9): 1145-1151. DOI:10.1016/j.resuscitation.2012.01.022 |
| [4] | Thannickal VJ, Toews GB. Mechanisms of pulmonary fibrosis[J]. Annu Rev Med, 2004, 55: 395-417. DOI:10.1146/annurev.med.55.091902.103810 |
| [5] | Coker RK, Laurent GJ, Shahzeidi S, et al. Transforming growth factor β1, -β2 and -β3 all stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis[J]. Am J Pathol, 1997, 150(3): 981-991. |
| [6] | Hoyt DG, Lazo JS. Early increases in pulmonary mRNA encoding procollagens and transforming growth factor-β precede bleomycin-induced pulmonary fibrosis in mice[J]. J Pharmacol Exp Ther, 1988, 246(2): 765-771. |
| [7] | Sime PJ, Xing Z, Graham FL, et al. Adenovector-mediated gene transfer of active transforming growth factor-b1 induces prolonged severe fibrosis in rat lung[J]. J Clin Invest, 1997, 100(4): 768-776. DOI:10.1172/JCI119590 |
| [8] | Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor b on bleomycin induced accumulation of lung collagen in mice[J]. Thorax, 1993, 48(10): 959-966. DOI:10.1136/thx.48.10.959 |
| [9] | Liu T, Chung MJ, Ullenbruch M, et al. Telomerase activity is required for bleomycin-induced pulmonary fibrosis in mice[J]. J Clin Invest, 2008, 117(12): 3800-3809. DOI:10.1172/JCI32369 |
| [10] | Luo F, Yan Z, Sides MD, et al. Arsenic trioxide inhibits transforming growth factor-β 1-induced fibroblast to myofibroblast differentiation in vitro and bleomycin induced lung fibrosis in vivo[J]. Resp Res, 2014, 15: 51. DOI:10.1186/1465-9921-15-51 |
| [11] | 黄敏, 齐小娟, 张平, 等. 结缔组织生长因子及α平滑肌肌动蛋白在PQ致大鼠肺纤维化中的作用[J]. 中华劳动卫生职业病杂志, 2010, 28(10): 729-734. DOI:10.3760/cma.j.issn.1001-9391.2010.10.006 |
| [12] | Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA[J]. Nature, 1990, 346(6284): 561-564. DOI:10.1038/346561a0 |
| [13] | Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids[J]. Nature, 1993, 365(6441): 61-65. DOI:10.1038/365061a0 |
| [14] | Smith SR, Terminelli C, Denhardt G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice[J]. J Pharmacol Exp Ther, 2000, 293: 136-150. |
| [15] | Wolf SA, Tauber S, Ullrich O. CNS immune surveillance and neuroinflammation: endocannabinoids keep control[J]. Curr Pharm Des, 2008, 14(23): 2266-2278. DOI:10.2174/138161208785740090 |
 2018, Vol. 27
2018, Vol. 27




