脓毒症是由宿主对感染反应失调进而引起的危及生命的器官功能障碍综合征[1-2]。脓毒症和脓毒性休克是全球威胁人类健康的主要医疗保健问题,2017年全球估计约发生4 890万例脓毒症,相关死亡约占全球总死亡率的19.7%[3-4]。近年来,尽管急诊与重症领域专家致力于研究脓毒症的发病机制和新型干预措施,但直至目前具体机制尚不明确,而对脓毒症患者的主要治疗方法仍然局限于抗生素和脏器支持治疗[5]。
微血管功能障碍是脓毒症的标志[6]。在脓毒症期间,血管内皮细胞向促凋亡、促炎、促黏连和促凝表型转变[7],损伤后可以导致血管通透性增加、组织水肿、白细胞黏附和血小板聚集增强、血管收缩功能丧失,与多器官功能障碍和临床不良结局相关[8]。然而,脓毒症导致血管内皮损伤的确切分子机制仍然知之甚少,特别是内皮细胞反应可能因器官而异,本综述旨在概述近年来对脓毒症相关血管内皮损伤的机制和新型干预措施,为进一步开展脓毒症的机制研究和临床治疗提供参考。
1 血管内皮的结构和功能 1.1 血管内皮的结构血管内皮细胞呈单层排列,形成血管的内表面。在人类中,内皮细胞表面积约270~7 000 m2[9]。内皮细胞管腔表面被糖萼包裹。糖萼由内皮细胞合成,具体成分包括蛋白聚糖骨架、糖胺聚糖侧链、糖蛋白和黏附的血浆蛋白,在止凝血、调节白细胞黏附以及感知机械力(如剪切应力和压力)等方面发挥关键作用;同时,糖萼还可以保护细胞表面受体,通过形成物理屏障阻止其激活[10]。复杂的内皮间连接结构,如黏附连接和紧密连接,在维持血管完整性方面起着至关重要的作用。黏附连接由核心跨膜蛋白血管内皮-钙黏蛋白(vascular-endothelial cadherin, VE-cadherin)组成,负责调节细胞-细胞黏附以及肌动蛋白细胞骨架和细胞内信号传导。紧密连接在细胞之间形成连续的细胞间屏障,控制离子和溶质的胞外运动。紧密连接由黏附分子组成,如claudin、occludin和连接黏附分子,与细胞质支架蛋白Zonula Occludens (ZO)-1、ZO-2和ZO-3形成复合物。连接蛋白是一种跨膜蛋白,形成细胞间通道,同时连接相邻细胞的细胞质,允许离子和代谢产物的交换,在维持血管稳态和细胞间通讯中发挥重要作用[9]。
1.2 血管内皮的功能屏障作用:大多数器官中毛细血管的连续内皮在血液和周围组织之间建立选择性屏障,对于维持体内平衡至关重要[11]。
细胞迁移:微血管形成了循环和不同器官间的关键通信接口[12],内皮细胞通过黏附分子、趋化因子和信号通路的复杂相互作用,调节细胞的生理稳态运输以及免疫细胞的刺激募集[13]。
血管通透性:内皮细胞在循环和周围组织之间形成调节通道[14],使水、气体和某些离子通过细胞内特定的通道、转运体或细胞间连接通过内皮细胞层。
组织灌注和血压:内皮细胞通过释放血管活性介质,作用于平滑肌细胞影响其收缩功能,在控制外周血管张力方面起着关键作用。血管内皮细胞分泌舒张因子主要包括一氧化氮(nitric oxide, NO)、前列腺素和内皮依赖性超极化因子等,收缩因子主要包括内皮素、血管紧张素等。
凝血:血管内皮可以维持一个连续的抗血栓形成的表面,但在需要时也可以立即触发凝血级联反应。为了对抗过度凝血反应,内皮细胞也具有不同的抗凝活性来维持凝血的平衡。
力学转导:血流都会对血管内皮细胞施加物理机械转导力,这种物理力量维持静止内皮的生理稳态[15]。内皮细胞可以通过受体酪氨酸激酶(VEGFR2和VEGFR3)、离子通道、整合素和连接蛋白(如PECAM-1和VE-cadherin)将机械刺激转化为生化信号[11]。
代谢界面:内皮细胞直接暴露于循环血液中,可以充当代谢界面[16],通过控制胰岛素、脂质和葡萄糖等物质的转运参与器官代谢的控制[17]。
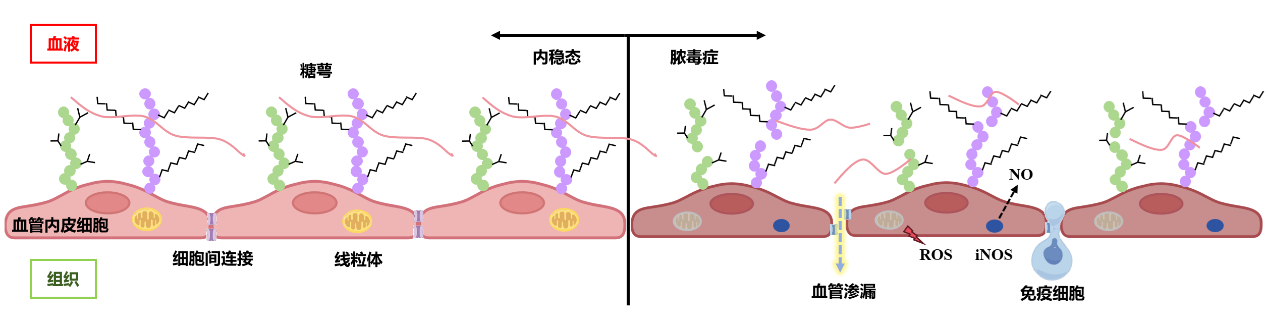
2 脓毒症导致血管内皮损伤的机制 2.1 糖萼降解在脓毒症中糖萼是内皮细胞最早发生损伤的部位[18]。急性损伤和炎症反应释放炎症因子(如TNF-α)和活性氧,激活脱落酶、肝素酶和金属蛋白酶等,介导切割糖萼的关键组成成分硫酸肝素和syndecan-1[19-20],透明质酸酶、凝血酶、弹性酶、纤溶酶原和组织蛋白酶B破坏糖萼的透明质酸[21]。糖萼结构脱落后黏附分子(如E-选择素和细胞间黏附分子-1)暴露在内皮之上,诱导白细胞黏附和血小板聚集,导致血栓形成,并与大量纤维蛋白结合,最终导致微循环功能障碍[21]。总的来说,糖萼成分的降解会导致血管渗漏、组织灌注受损、凝血功能异常、白细胞活化和黏附[10]。
2.2 黏附连接和紧密连接破坏既往研究发现,在脓毒症中,黏附连接VE-cadherin的胞外结构受到中性粒细胞弹性蛋白酶和金属蛋白酶的蛋白水解,血管内皮生长因子(vascular endothelial growth factor, VEGF)也会导致VE-cadherin和occludin的丢失[9]。对脓毒症患者尸检发现脑组织中微血管内皮细胞的紧密连接蛋白occludin、claudin-5和ZO-1显著下调[22]。多种病原体、细菌毒素和细胞因子可诱导中性粒细胞细胞捕获网(neutrophil extracellular traps, NETs)形成。NETs可以降低VE-cadherin和ZO-1蛋白的表达[23],白细胞和内皮细胞产生的白介素6也可以降低VE-cadherin和ZO-1的表达,导致血管屏障功能障碍[24]。
2.3 线粒体功能障碍线粒体是维持血管稳态的重要参与者。作为氧化应激的主要场所,线粒体在炎症反应中产生活性氧增加线粒体分裂,受损线粒体释放的线粒体DNA最终引起Z-DNA结合蛋白1介导的内皮细胞泛凋亡PANoptosis[25]。
2.4 血管张力变化血管内皮细胞通过分泌舒张/收缩因子调节血管张力,是维持微循环血流完整性的重要因素。NO是内皮细胞通过一氧化氮合酶(nitric oxide synthase, NOS)代谢L-精氨酸而产生的,发挥舒张血管的作用,人体内有3种不同的NOS亚型,即神经元型NOS(neuronal-type nitric oxide synthase, nNOS)、内皮型NOS(endothelial nitric oxide synthase, eNOS)、诱导型NOS(inducible nitric oxide synthase, iNOS),其产生受内皮剪切应力和各种信号分子的调节,如缓激肽、腺苷、血清素和VEGF[9]。在正常生理状态下,血管内皮细胞平衡iNOS/eNOS-NO体系,以eNOS生成NO维持血管的张力。iNOS在健康机体中基本不表达,当受到炎症刺激时大量表达。既往研究发现,在脓毒症期间,机体大量产生iNOS,各种组织和细胞产生过量NO而产生强烈的舒张血管作用,随后影响微循环血流灌注。此外,NO在微血管腔内可以调节红细胞和白细胞变形能力,以及血小板黏附和聚集。NO的过量与血压降低、微血管反应性受损、红细胞变形能力异常、功能毛细血管密度降低和氧消耗减少有关[26]。
3 血管内皮损伤与多器官功能障碍机体在遭受脓毒症打击后出现进行性器官功能障碍,当两个或多个器官同时或序贯受到影响时,可导致多器官功能障碍综合征(multiple organ dysfunction syndrome, MODS)。血管内皮细胞损伤和微血管功能障碍是导致MODS的重要原因[27-28]。血管内皮细胞在不同的器官中具有共性,也具有异质性,这可能是导致脓毒症表型多样性的原因之一。血管内皮在结构和分子上以器官形式分化,使其能够执行特异性的器官和微环境功能[11]。脑、肺或肌肉组织具有紧密连接的连续内皮细胞,内皮细胞在其中发挥屏障功能;具有过滤功能的器官(如肾脏)或内分泌腺器官存在不连续的内皮;免疫活性器官,如骨骼、脾脏和肝脏中由窦状内皮形成不连续的细胞层,细胞内和细胞间有筛状间隙[11, 27]。紧密连接和黏附连接的分布在不同器官中存在差异,紧密连接在血脑屏障和脑微循环中高度表达,肺内皮比肾脏和肝脏表现出更高的黏附分子表达和更高的通透性[29]。见图 1。
|
| 图 1 脓毒症导致血管内皮损伤的机制 |
|
|
肾脏微血管内皮细胞在脓毒症病理生理中起重要作用[30]。肾微血管与其他器官有许多共同的功能,但是肾皮质小动脉、肾小球、小管周围毛细血管和毛细血管后小静脉中的微血管内皮细胞也具有内在的分子和表型异质性,并以段特异性的方式对脓毒症诱导的AKI做出反应[30]。脓毒症时,肾脏传出小动脉血管扩张和肾内分流导致大血管功能障碍。大血管功能障碍使肾血流从髓质转向皮质,导致髓质灌注和氧合减少,进一步恶化肾功能[31]。对于微血管系统,脓毒症时促炎细胞因子升高和白细胞活化可能导致肾毛细血管形成微血栓,微血栓的形成导致血流减少[32]。这些血管功能障碍会导致活性氧的产生,进一步破坏内皮屏障,最终导致内皮渗漏[33]。
3.2 脓毒症急性肺损伤(acute lung injury, ALI)/急性呼吸窘迫综合征(acute respiratory distress syndrome, ARDS)脓毒症相关ALI/ARDS是导致患者死亡的重要原因,内皮细胞激活在其发病机制中起重要作用[34]。ALI/ARDS的肺泡毛细血管损伤是微生物和宿主因子对肺泡毛细血管直接和间接作用的结果[35]。三结构域蛋白(tripartite motif-containing protein, TRIM)是E3泛素连接酶的一个亚族,参与细胞增殖和分化、先天免疫和自噬等过程,TRIM47作为一种新的内皮细胞激活剂,在脓毒症中通过激活内皮细胞TRAF2-MAPK/NF-κB促炎轴介导炎症反应,促进内皮细胞炎症和ALI[36]。在炎症和溶血条件下血红蛋白介导肺血管损伤,血红蛋白增加白细胞内皮黏附并通过TLR4信号激活人肺微血管内皮细胞可能是潜在的机制[37]。VEGF是血管通透性的主要调节因子,肺血管内皮细胞通过多种MAPK依赖途径诱导VEGF分泌增加,促进ALI中非心源性肺水肿的发展[38]。
3.3 脓毒症相关脑病脓毒症相关脑病是由脓毒症引起的脑弥漫性功能障碍。脑微血管内皮细胞是血脑屏障(blood-brain barrier, BBB)的主要组成部分。BBB的作用是调节神经系统的微环境,控制通过脑毛细血管的血液流动,并防止血液循环中有害物质的流入。脓毒症发病过程中与血脑屏障破坏有关的病理生理因素包括VEGF的上调和VEGFR2的激活、黏附连接的紊乱、紧密连接蛋白的表达减少、炎症细胞因子的激活或上调、氧化应激诱导和基质金属蛋白酶的上调[39]。这些因素可能协同作用,破坏血脑屏障的通透性,导致神经元损伤[40]。此外,线粒体裂解主要由动力相关蛋白1(dynamin-related protein 1, DRP1)、线粒体分裂蛋白1(mito-Fission 1, FIS1)介导,研究发现通过DRP1-FIS1相互作用介导的线粒体功能障碍与脓毒症血脑屏障紧密连接缺失和通透性增加有关[41]。
3.4 脓毒症相关心肌损伤内皮功能障碍介导的心肌水肿在脓毒性心肌病中起重要作用[42],然而其机制尚不清楚。既往研究发现CD1依赖性自然杀伤T细胞(natural killer T, NKT)的缺失可以导致脓毒症小鼠发生心功能障碍、内皮细胞凋亡、微血管损伤、微血管通透性和心脏水肿增加,可能与心肌T淋巴细胞的聚集和白介素6蛋白水平升高有关[42]。DNA依赖性蛋白激酶催化亚基(DNA-dependent protein kinase catalytic subunit, DNA-PKcs)在脓毒症中促进病理性线粒体分裂,线粒体衍生肽MOTS-c具有抗炎活性,DNA-PKcs失活可以恢复MOTS-c表达,防止c-Jun N-terminal Kinase (JNK)诱导的抑制蛋白磷酸化,改善F-肌动蛋白聚合,增强伪足完整性,最终保护内皮屏障功能,减轻心肌微血管损伤[43]。
4 治疗血管内皮细胞损伤在脓毒症的发展中起到重要作用,其关键的治疗目标是保存和恢复血管内皮功能,主要的治疗方法集中于减轻内皮结构损伤、抑制炎症反应或减少微血管血栓形成[44]。虽然基础研究方面取得了相应好的结果,但是截至目前,在大型临床试验中,并没有能显著提高脓毒症患者生存率的治疗方法[7, 45],这可能与脓毒症的异质性有关[46]。
4.1 稳定糖萼内皮糖萼损伤与多器官功能障碍有关,靶向保护糖萼功能正在成为脓毒症的潜在治疗策略[28]。既往研究发现乌司他丁可以抑制肺血管内皮肝素酶活性并减轻糖萼损伤[47]。一种线粒体肽的合成衍生物Colivelin具有恢复内皮功能和减轻糖萼结构损伤的功能[10]。重组人血栓调节蛋白通过保护内皮糖萼减轻脓毒症引起的心肌功能障碍和ARDS[48-49]。此外,氢化可的松也可以发挥保护内皮糖萼的功能[50]。血浆中含有一些生物活性成分可能与糖萼存在相互作用[28],其中输注白蛋白可能是减少糖萼损伤的有效方法[51]。鞘氨醇-1-磷酸介导白蛋白和其他血浆蛋白(如高密度脂蛋白相关载脂蛋白M)减少内皮糖萼脱落从而稳定糖萼[52-53]。血浆丝氨酸蛋白酶如抗凝血酶也可能保护糖萼的完整性,但其作用机制需要进一步研究来阐明[50]。因此,血浆输注可能作为保护糖萼损伤的潜在治疗方法[28]。
4.2 保护细胞间连接前蛋白转化酶枯草溶菌素9(proprotein convertase Subtilisin/Kexin-9, PCSK-9)水平在脓毒症患者中升高[54],在体外和体内脓毒症模型中观察到抑制PCSK-9可以增加VE-cadherin表达[9, 55]。腺苷单磷酸激活的蛋白激酶(α1AMP-activated protein kinase, α1AMPK)通过维持内皮间紧密连接减轻心室壁水肿[56]。卡格列净是一种钠-葡萄糖共转运蛋白2抑制剂,通过激活内皮细胞中的AMPK发挥内皮保护作用而减轻脓毒症毛细血管渗漏[57]。乌司他丁和TNF-α受体拮抗剂可逆转脓毒症中内皮连接蛋白occludin、claudin-5和ZO-1的表达降低[58]。普伐他汀可以上调肺微血管内皮细胞连接蛋白ZO-1、JAM-C和VE-cadherin的水平[59],改善肺泡内皮屏障破坏。利格列汀可以以剂量依赖的方式减轻脓毒症肺微血管内皮细胞高通透性和细胞间连接VE-cadherin,β-catenin和ZO-1的破坏[60]。食欲素B是一种源自下丘脑外侧食欲素神经元的神经肽,具有多种生物学功能,研究发现食欲素B通过增加紧密连接蛋白ZO-1和occludin的表达减轻脓毒症小鼠肺组织血管内皮的通透性[61]。间充质基质细胞是一种多能干细胞,可以分化为多种间充质细胞系的细胞类型,MSC治疗可以增加VE-cadherin表达,并增强VE-cadherin和β-catenin向质膜的募集,从而降低内皮细胞的通透性[62]。垂体中叶素通过促进VE-cadherin的重新定位,动态修复损坏的内皮连接[63]。
4.3 减少NO释放选择性阻断iNOS活性、减少NO释放可能改善脓毒症微血管障碍[7]。亚甲基蓝是NO通路抑制剂,通过与可溶性鸟苷酸环化酶结合,阻断环鸟苷单磷酸的产生,发挥抑制NO合成酶和清除NO的作用[64]。胍丁胺可以抑制iNOS表达,减轻血管氧化应激和内皮损伤[65]。occludin去磷酸化会导致紧密连接破坏和血管通透性增加,在脓毒症模型中发现维生素C通过阻止occludin去磷酸化,阻止eNOS解偶联,降低iNOS活性,减少NO和活性氧的过量产生,减轻血管渗漏[66]。然而,使用iNOS抑制剂治疗并不能改善脓毒症患者的预后,甚至可能存在血流动力学紊乱的风险[7]。内皮钙蛋白酶通过抑制p38磷酸化从而减弱iNOS的表达,进一步减少NO和活性氧过度产生诱导的内皮细胞凋亡,在脓毒症相关AKI中发挥保护作用[67]。
4.4 中医中药祖国医学在治疗脓毒症相关血管内皮损伤方面有巨大潜力。在脓毒症患者中,参附注射液可以显著降低血浆中内皮损伤标志物血管生成素-2和Syndecan-1的水平;在小鼠脓毒症模型中发现,参附注射液抑制内皮黏附分子的上调,同时恢复肺、肾、肝组织血管中VE-cadherin的表达[68]。血必净注射液通过抑制eNOS脱耦联减轻脓毒症血管内皮细胞氧化应激[69]。丹参酮ⅡA可能通过抑制NF-κB信号通路降低血管内皮细胞凋亡发挥对脓毒症大鼠血管内皮细胞的保护作用[70]。热毒宁可以通过减少肺微血管内皮细胞的凋亡来减轻脓毒症相关急性肺损伤[71]。
4.5 其他干预措施除上述治疗,近年来一些其他的干预措施也发现具有血管内皮保护作用。研究发现伊马替尼可以保护内皮细胞超微结构和内皮屏障功能,减少血管渗漏,保持微循环灌注和氧合[72]。选择性加压素V1A受体激动剂Selepressin可以降低心肌和肺组织中VEGF和Ang-2浓度[73],但是并没有改善脓毒性休克患者的临床结果[74]。肾上腺髓质素是内皮屏障功能障碍的重要调节因子,人源化重组单克隆抗肾上腺髓质素抗体HAM8101 (Adrecizumab),显著降低VEGF的表达,改善血管屏障功能[75]。雷贝拉唑是一种有效的HIF-1α诱导剂,通过内皮HIF-1α/FoxM1信号通路促进血管修复,改善脓毒症急性肺损伤[76]。运动训练对脓毒症导致的多脏器功能障碍具有保护作用,R-spondin (RSPO)蛋白家族是一组分泌蛋白,在血管内皮功能调节中发挥生物学功能,有氧运动通过增加R-spondin 3 (RSPO3)的表达,抑制基质金属蛋白酶介导的糖萼、紧密连接和黏附连接的破坏,保护肾内皮高通透性和脓毒症相关AKI[77]。
5 结语血管内皮损伤在脓毒症微血管功能障碍和多器官功能衰竭中起着重要作用。脓毒症相关血管内皮损伤的分子机制是复杂的,在不同器官存在一定的异质性,也导致干预措施的效果不一。未来的研究应着力于明确脓毒症异质性的原因,探索血管内皮损伤的不同表征,可能有助于靶向治疗脓毒症,改善患者预后。
利益冲突 所有作者声明无利益冲突
本文思维导图请登陆中华急诊网(www.cem.org.cn)浏览(Html格式全文)
| [1] | Chen AX, Simpson SQ, Pallin DJ. Sepsis guidelines[J]. N Engl J Med, 2019, 380(14): 1369-1371. DOI:10.1056/NEJMclde1815472 |
| [2] | Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3)[J]. JAMA, 2016, 315(8): 801-810. DOI:10.1001/jama.2016.0287 |
| [3] | Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study[J]. Lancet, 2020, 395(10219): 200-211. DOI:10.1016/S0140-6736(19)32989-7 |
| [4] | Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021[J]. Crit Care Med, 2021, 49(11): e1063-e1143. DOI:10.1097/ccm.0000000000005337 |
| [5] | Cohen J, Vincent JL, Adhikari NKJ, et al. Sepsis: a roadmap for future research[J]. Lancet Infect Dis, 2015, 15(5): 581-614. DOI:10.1016/S1473-3099(15)70112-X |
| [6] | Ince C, Boerma EC, Cecconi M, et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European Society of Intensive Care Medicine[J]. Intensive Care Med, 2018, 44(3): 281-299. DOI:10.1007/s00134-018-5070-7 |
| [7] | Joffre J, Hellman J, Ince C, et al. Endothelial responses in sepsis[J]. Am J Respir Crit Care Med, 2020, 202(3): 361-370. DOI:10.1164/rccm.201910-1911TR |
| [8] | Joffre J, Hellman J. Oxidative stress and endothelial dysfunction in sepsis and acute inflammation[J]. Antioxid Redox Signal, 2021, 35(15): 1291-1307. DOI:10.1089/ars.2021.0027 |
| [9] | McMullan RR, McAuley DF, O'Kane CM, et al. Vascular leak in sepsis: physiological basis and potential therapeutic advances[J]. Crit Care, 2024, 28(1): 97. DOI:10.1186/s13054-024-04875-6 |
| [10] | Urban C, Hayes HV, Piraino G, et al. Colivelin, a synthetic derivative of humanin, ameliorates endothelial injury and glycocalyx shedding after sepsis in mice[J]. Front Immunol, 2022, 13: 984298. DOI:10.3389/fimmu.2022.984298 |
| [11] | G A, Young K. A systems view of the vascular endothelium in health and disease[J]. Cell, 2024, 187(18): 4833-4858. DOI:10.1016/j.cell.2024.07.012 |
| [12] | Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated[J]. Nat Rev Immunol, 2007, 7(9): 678-689. DOI:10.1038/nri2156 |
| [13] | Vestweber D. How leukocytes cross the vascular endothelium[J]. Nat Rev Immunol, 2015, 15(11): 692-704. DOI:10.1038/nri3908 |
| [14] | Claesson-Welsh L, Dejana E, McDonald DM. Permeability of the endothelial barrier: identifying and reconciling controversies[J]. Trends Mol Med, 2021, 27(4): 314-331. DOI:10.1016/j.molmed.2020.11.006 |
| [15] | Dominguez A, Iruela-Arispe ML. Integration of Chemo-mechanical signaling in response to fluid shear stress by the endothelium[J]. Curr Opin Cell Biol, 2023, 85: 102232. DOI:10.1016/j.ceb.2023.102232 |
| [16] | Kalucka J, de Rooij LPMH, Goveia J, et al. Single-cell transcriptome atlas of murine endothelial cells[J]. Cell, 2020, 180(4): 764-779. DOI:10.1016/j.cell.2020.01.015 |
| [17] | Hasan SS, Fischer A. The endothelium: an active regulator of lipid and glucose homeostasis[J]. Trends Cell Biol, 2021, 31(1): 37-49. DOI:10.1016/j.tcb.2020.10.003 |
| [18] | Chelazzi C, Villa G, Mancinelli P, et al. Glycocalyx and sepsis-induced alterations in vascular permeability[J]. Crit Care, 2015, 19(1): 26. DOI:10.1186/s13054-015-0741-z |
| [19] | Schmidt EP, Yang YM, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis[J]. Nat Med, 2012, 18(8): 1217-1223. DOI:10.1038/nm.2843 |
| [20] | Manon-Jensen T, Multhaupt HAB, Couchman JR. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains[J]. FEBS J, 2013, 280(10): 2320-2331. DOI:10.1111/febs.12174 |
| [21] | Iba T, Levy JH. Derangement of the endothelial glycocalyx in sepsis[J]. J Thromb Haemost, 2019, 17(2): 283-294. DOI:10.1111/jth.14371 |
| [22] | Erikson K, Tuominen H, Vakkala M, et al. Brain tight junction protein expression in sepsis in an autopsy series[J]. Crit Care, 2020, 24(1): 385. DOI:10.1186/s13054-020-03101-3 |
| [23] | Maneta E, Aivalioti E, Tual-Chalot S, et al. Endothelial dysfunction and immunothrombosis in sepsis[J]. Front Immunol, 2023, 14: 1144229. DOI:10.3389/fimmu.2023.1144229 |
| [24] | Alsaffar H, Martino N, Garrett JP, et al. Interleukin-6 promotes a sustained loss of endothelial barrier function via Janus kinase-mediated STAT3 phosphorylation and de novo protein synthesis[J]. Am J Physiol Cell Physiol, 2018, 314(5): C589-C602. DOI:10.1152/ajpcell.00235.2017 |
| [25] | Wang Y, Shi YX, Shao YW, et al. S100A8/A9(hi) neutrophils induce mitochondrial dysfunction and PANoptosis in endothelial cells via mitochondrial complex Ⅰ deficiency during sepsis[J]. Cell Death Dis, 2024, 15(6): 462. DOI:10.1038/s41419-024-06849-6 |
| [26] | Bateman RM, Sharpe MD, Ellis CG. Bench-to-bedside review: microvascular dysfunction in sepsis: hemodynamics, oxygen transport, and nitric oxide[J]. Crit Care, 2003, 7(5): 359-373. DOI:10.1186/cc2353 |
| [27] | Cleuren A, Molema G. Organotypic heterogeneity in microvascular endothelial cell responses in sepsis-a molecular treasure trove and pharmacological Gordian knot[J]. Front Med, 2023, 10: 1252021. DOI:10.3389/fmed.2023.1252021 |
| [28] | Kravitz MS, Kattouf N, Stewart IJ, et al. Plasma for prevention and treatment of glycocalyx degradation in trauma and sepsis[J]. Crit Care, 2024, 28(1): 254. DOI:10.1186/s13054-024-05026-7 |
| [29] | Ince C, Mayeux PR, Nguyen T, et al. The endothelium in sepsis[J]. Shock, 2016, 45(3): 259-270. DOI:10.1097/shk.0000000000000473 |
| [30] | Molema G, Zijlstra JG, van Meurs M, et al. Renal microvascular endothelial cell responses in sepsis-induced acute kidney injury[J]. Nat Rev Nephrol, 2022, 18(2): 95-112. DOI:10.1038/s41581-021-00489-1 |
| [31] | Calzavacca P, Evans RG, Bailey M, et al. Variable responses of regional renal oxygenation and perfusion to vasoactive agents in awake sheep[J]. Am J Physiol Regul Integr Comp Physiol, 2015, 309(10): R1226-R1233. DOI:10.1152/ajpregu.00228.2015 |
| [32] | Post EH, Kellum JA, Bellomo R, et al. Renal perfusion in sepsis: from macro- to microcirculation[J]. Kidney Int, 2017, 91(1): 45-60. DOI:10.1016/j.kint.2016.07.032 |
| [33] | Chang YM, Chou YT, Kan WC, et al. Sepsis and acute kidney injury: a review focusing on the bidirectional interplay[J]. Int J Mol Sci, 2022, 23(16): 9159. DOI:10.3390/ijms23169159 |
| [34] | Cusack R, Bos LD, Povoa P, et al. Endothelial dysfunction triggers acute respiratory distress syndrome in patients with sepsis: a narrative review[J]. Front Med, 2023, 10: 1203827. DOI:10.3389/fmed.2023.1203827 |
| [35] | Dos Santos CC, Amatullah H, Vaswani CM, et al. Mesenchymal stromal (stem) cell therapy modulates miR-193b-5p expression to attenuate sepsis-induced acute lung injury[J]. Eur Respir J, 2022, 59(1). DOI:10.1183/13993003.04216-2020 |
| [36] | Qian YS, Wang ZW, Lin HR, et al. TRIM47 is a novel endothelial activation factor that aggravates lipopolysaccharide-induced acute lung injury in mice via K63-linked ubiquitination of TRAF2[J]. Signal Transduct Target Ther, 2022, 7(1): 148. DOI:10.1038/s41392-022-00953-9 |
| [37] | Conger AK, Tomasek T, Riedmann KJ, et al. Hemoglobin increases leukocyte adhesion and initiates lung microvascular endothelial activation via Toll-like receptor 4 signaling[J]. Am J Physiol Cell Physiol, 2023, 324(3): C665-C673. DOI:10.1152/ajpcell.00211.2022 |
| [38] | Tomita K, Saito Y, Suzuki T, et al. Vascular endothelial growth factor contributes to lung vascular hyperpermeability in sepsis-associated acute lung injury[J]. Naunyn Schmiedebergs Arch Pharmacol, 2020, 393(12): 2365-2374. DOI:10.1007/s00210-020-01947-6 |
| [39] | Archie SR, Al Shoyaib A, Cucullo L. Blood-brain barrier dysfunction in CNS disorders and putative therapeutic targets: an overview[J]. Pharmaceutics, 2021, 13(11): 1779. DOI:10.3390/pharmaceutics13111779 |
| [40] | Dumbuya JS, Li SQ, Liang LL, et al. Paediatric sepsis-associated encephalopathy (SAE): a comprehensive review[J]. Mol Med, 2023, 29(1): 27. DOI:10.1186/s10020-023-00621-w |
| [41] | Haileselassie B, Joshi AU, Minhas PS, et al. Mitochondrial dysfunction mediated through dynamin-related protein 1 (Drp1) propagates impairment in blood brain barrier in septic encephalopathy[J]. J Neuroinflammation, 2020, 17(1): 36. DOI:10.1186/s12974-019-1689-8 |
| [42] | Chen LR, Yang H, Liu L, et al. CD1d-dependent natural killer T-cells inactivation aggravates sepsis-induced myocardial injury via T lymphocytes infiltration and IL-6 production in mice[J]. Int Immunopharmacol, 2023, 120: 110256. DOI:10.1016/j.intimp.2023.110256 |
| [43] | Zou RJ, Shi WT, Chang X, et al. The DNA-dependent protein kinase catalytic subunit exacerbates endotoxemia-induced myocardial microvascular injury by disrupting the MOTS-c/JNK pathway and inducing profilin-mediated lamellipodia degradation[J]. Theranostics, 2024, 14(4): 1561-1582. DOI:10.7150/thno.92650 |
| [44] | Cavaillon JM, Singer M, Skirecki T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads[J]. EMBO Mol Med, 2020, 12(4): e10128. DOI:10.15252/emmm.201810128 |
| [45] | Grimaldi D, Vincent JL. Clinical trial research in focus: rethinking trials in sepsis[J]. Lancet Respir Med, 2017, 5(8): 610-611. DOI:10.1016/S2213-2600(17)30268-0 |
| [46] | Alfano DN, Miller MJ, Bubeck Wardenburg J. Endothelial ADAM10 utilization defines a molecular pathway of vascular injury in mice with bacterial sepsis[J]. J Clin Invest, 2023, 133(23): e168450. DOI:10.1172/JCI168450 |
| [47] | Wang LP, Huang X, Kong GQ, et al. Ulinastatin attenuates pulmonary endothelial glycocalyx damage and inhibits endothelial heparanase activity in LPS-induced ARDS[J]. Biochem Biophys Res Commun, 2016, 478(2): 669-675. DOI:10.1016/j.bbrc.2016.08.005 |
| [48] | Kakino Y, Doi T, Okada H, et al. Recombinant thrombomodulin may protect cardiac capillary endothelial glycocalyx through promoting Glypican-1 expression under experimental endotoxemia[J]. Heliyon, 2022, 8(11): e11262. DOI:10.1016/j.heliyon.2022.e11262 |
| [49] | Suzuki K, Okada H, Takemura G, et al. Recombinant thrombomodulin protects against LPS-induced acute respiratory distress syndrome via preservation of pulmonary endothelial glycocalyx[J]. Br J Pharmacol, 2020, 177(17): 4021-4033. DOI:10.1111/bph.15153 |
| [50] | Chappell D, Hofmann-Kiefer K, Jacob M, et al. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin[J]. Basic Res Cardiol, 2009, 104(1): 78-89. DOI:10.1007/s00395-008-0749-5 |
| [51] | Becker BF, Jacob M, Leipert S, et al. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases[J]. Br J Clin Pharmacol, 2015, 80(3): 389-402. DOI:10.1111/bcp.12629 |
| [52] | Zeng Y, Adamson RH, Curry FE, et al. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding[J]. Am J Physiol Heart Circ Physiol, 2014, 306(3): H363-H372. DOI:10.1152/ajpheart.00687.2013 |
| [53] | Christensen PM, Liu CH, Swendeman SL, et al. Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1[J]. FASEB J, 2016, 30(6): 2351-2359. DOI:10.1096/fj.201500064 |
| [54] | Innocenti F, Gori AM, Giusti B, et al. Plasma PCSK9 levels and sepsis severity: an early assessment in the emergency department[J]. Clin Exp Med, 2021, 21(1): 101-107. DOI:10.1007/s10238-020-00658-9 |
| [55] | Huang LX, Li YJ, Cheng Z, et al. PCSK9 promotes endothelial dysfunction during sepsis via the TLR4/MyD88/NF-κB and NLRP3 pathways[J]. Inflammation, 2023, 46(1): 115-128. DOI:10.1007/s10753-022-01715-z |
| [56] | Castanares-Zapatero D, Bouleti C, Sommereyns C, et al. Connection between cardiac vascular permeability, myocardial edema, and inflammation during sepsis: role of the α1AMP-activated protein kinase isoform[J]. Crit Care Med, 2013, 41(12): e411-22. DOI:10.1097/CCM.0b013e31829866dc |
| [57] | Angé M, De Poortere J, Ginion A, et al. Canagliflozin protects against sepsis capillary leak syndrome by activating endothelial α1AMPK[J]. Sci Rep, 2021, 11(1): 13700. DOI:10.1038/s41598-021-93156-1 |
| [58] | Fang M, Zhong WH, Song WL, et al. Ulinastatin ameliorates pulmonary capillary endothelial permeability induced by sepsis through protection of tight junctions via inhibition of TNF-α and related pathways[J]. Front Pharmacol, 2018, 9: 823. DOI:10.3389/fphar.2018.00823 |
| [59] | Ren Y, Li L, Wang MM, et al. Pravastatin attenuates sepsis-induced acute lung injury through decreasing pulmonary microvascular permeability via inhibition of Cav-1/ENOS pathway[J]. Int Immunopharmacol, 2021, 100: 108077. DOI:10.1016/j.intimp.2021.108077 |
| [60] | Zhang N, Tang SH, Zhang JJ, et al. The dipeptidyl peptidase-4 inhibitor linagliptin ameliorates LPS-induced acute lung injury by maintenance of pulmonary microvascular barrier via activating the Epac1/AKT pathway[J]. Biomed Pharmacother, 2022, 155: 113704. DOI:10.1016/j.biopha.2022.113704 |
| [61] | Wang YY, Wan XH, Li YS. Orexin B alleviates sepsis-associated lung injury through the attenuation of pulmonary endothelial barrier dysfunction by regulating the rho-associated coiled-coil containing protein kinase 2/zonula occludens-1 (ROCK2/ZO-1) axis[J]. Biotechnol Appl Biochem, 2025, 72(4): 883-896. DOI:10.1002/bab.2703 |
| [62] | Pati S, Khakoo AY, Zhao J, et al. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/β-catenin signaling[J]. Stem Cells Dev, 2011, 20(1): 89-101. DOI:10.1089/scd.2010.0013 |
| [63] | Xiao F, Wang DN, Kong LM, et al. Intermedin protects against sepsis by concurrently re-establishing the endothelial barrier and alleviating inflammatory responses[J]. Nat Commun, 2018, 9(1): 2644. DOI:10.1038/s41467-018-05062-2 |
| [64] | Heemskerk S, van Haren FMP, Foudraine NA, et al. Short-term beneficial effects of methylene blue on kidney damage in septic shock patients[J]. Intensive Care Med, 2008, 34(2): 350-354. DOI:10.1007/s00134-007-0867-9 |
| [65] | El-Awady MS, Nader MA, Sharawy MH. The inhibition of inducible nitric oxide synthase and oxidative stress by agmatine attenuates vascular dysfunction in rat acute endotoxemic model[J]. Environ Toxicol Pharmacol, 2017, 55: 74-80. DOI:10.1016/j.etap.2017.08.009 |
| [66] | Zhou G, Kamenos G, Pendem S, et al. Ascorbate protects against vascular leakage in cecal ligation and puncture-induced septic peritonitis[J]. Am J Physiol Regul Integr Comp Physiol, 2012, 302(4): R409-R416. DOI:10.1152/ajpregu.00153.2011 |
| [67] | Liu ZF, Ji JJ, Zheng D, et al. Protective role of endothelial calpain knockout in lipopolysaccharide-induced acute kidney injury via attenuation of the p38-iNOS pathway and NO/ROS production[J]. Exp Mol Med, 2020, 52(4): 702-712. DOI:10.1038/s12276-020-0426-9 |
| [68] | Tian R, Li RR, Chen Y, et al. Shenfu injection ameliorates endotoxemia-associated endothelial dysfunction and organ injury via inhibiting PI3K/Akt-mediated glycolysis[J]. J Ethnopharmacol, 2024, 335: 118634. DOI:10.1016/j.jep.2024.118634 |
| [69] | 张晓菲, 刘英, 王丽纯, 等. 血必净注射液抑制eNOS脱耦联减轻脓毒症血管内皮细胞氧化应激[J]. 广州医科大学学报, 2020, 48(6): 29-32. DOI:10.3969/j.issn.2095-9664.2020.06.07 |
| [70] | 刘旭东, 冯俊, 周代星, 等. 丹参酮ⅡA通过调控NF-κB信号通路对脓毒症大鼠血管内皮细胞的保护作用[J]. 疑难病杂志, 2021, 20(9): 935-938. DOI:10.3969/j.issn.1671-6450.2021.09.015 |
| [71] | Wang ZY, Guo Z, Wang XS, et al. Reduning alleviates sepsis-induced acute lung injury by reducing apoptosis of pulmonary microvascular endothelial cells[J]. Front Immunol, 2023, 14: 1196350. DOI:10.3389/fimmu.2023.1196350 |
| [72] | Koning NJ, de Lange F, van Meurs M, et al. Reduction of vascular leakage by imatinib is associated with preserved microcirculatory perfusion and reduced renal injury markers in a rat model of cardiopulmonary bypass[J]. Br J Anaesth, 2018, 120(6): 1165-1175. DOI:10.1016/j.bja.2017.11.095 |
| [73] | Rehberg S, Yamamoto Y, Sousse L, et al. Selective Ⅴ(1a) agonism attenuates vascular dysfunction and fluid accumulation in ovine severe sepsis[J]. Am J Physiol Heart Circ Physiol, 2012, 303(10): H1245-H1254. DOI:10.1152/ajpheart.00390.2012 |
| [74] | Laterre PF, Berry SM, Blemings A, et al. Effect of selepressin vs placebo on ventilator- and vasopressor-free days in patients with septic shock: the SEPSIS-ACT randomized clinical trial[J]. JAMA, 2019, 322(15): 1476-1485. DOI:10.1001/jama.2019.14607 |
| [75] | Geven C, Peters E, Schroedter M, et al. Effects of the humanized anti-adrenomedullin antibody adrecizumab (HAM8101) on vascular barrier function and survival in rodent models of systemic inflammation and sepsis[J]. Shock, 2018, 50(6): 648-654. DOI:10.1097/SHK.0000000000001102 |
| [76] | Evans CE, Peng Y, Zhu MM, et al. Rabeprazole promotes vascular repair and resolution of sepsis-induced inflammatory lung injury through HIF-1α[J]. Cells, 2022, 11(9): 1425. DOI:10.3390/cells11091425 |
| [77] | Xu QF, Zhang H, Zhao Y, et al. Increased R-spondin 3 contributes to aerobic exercise-induced protection against renal vascular endothelial hyperpermeability and acute kidney injury[J]. Acta Physiol, 2023, 239(4): e14036. DOI:10.1111/apha.14036 |
 2026, Vol. 35
2026, Vol. 35