2. 青海大学临床医学院,西宁 810001
心脏骤停的发病率在全球范围内持续上升,是重大的公共卫生问题。心脏骤停导致全身组织器官缺血缺氧,自主循环恢复(return of spontaneous circulation, ROSC)后由于缺血-再灌注损伤可导致多器官功能障碍,其中心脏骤停ROSC后发生的脑功能障碍称为心脏骤停后脑损伤(post-cardiac arrest brain injury, PCABI)。虽然近几年我国心脏骤停患者复苏的成功率较前升高,但神经功能的预后并未得到显著改善[1],而PCABI恰好是心脏骤停患者ROSC后死亡的重要原因之一,也是心脏骤停幸存者遗留长期功能残疾的首要原因[2],因此,为了提高心脏骤停幸存患者的生存率和生活质量,寻找到有效的治疗手段来缓解PCABI的进展迫在眉睫。
心脏骤停ROSC后缺血-再灌注可以激活脑内无菌性炎症反应,多种免疫细胞和免疫因子共同参与,引起脑组织免疫稳态失衡,导致神经元受损介导PCABI的发生发展。近几年免疫调节PCABI进展受到越来越多的关注,也使研究者意识到了调节免疫对脑保护的重要性。因此,为了进一步寻找PCABI有效的治疗手段,近几年诸多学者对免疫系统影响PCABI的机制进行了更为深入的研究并取得了新的进展,本文对近几年的相关研究进行了综述,期望为进一步阐明PCABI的发生机制和未来治疗方向提供新的思路。
1 PCABI的病理生理学PCABI主要包括心脏骤停发生时的缺血性损伤和ROSC后的再灌注损伤[3]。心脏骤停后因缺血缺氧导致脑细胞线粒体氧化磷酸化障碍,三磷酸腺苷(adenosine triphosphate, ATP)生成障碍,当ATP耗竭后细胞膜质子泵Na+外排障碍引起细胞水肿,严重时形成脑水肿,另一方面由ATP耗竭后引起的自由基生成增多能够直接导致细胞损伤。再灌注后细胞膜Na+-Ca2+交换引起Ca2+内流,线粒体和内质网膜损伤后大量Ca2+也流入细胞内,造成细胞内钙超载,进而激活多种蛋白酶和磷脂酶等促进细胞多种功能和结构蛋白分解,进一步加重神经元损伤[4]。
缺血缺氧引起的细胞膜膜蛋白的降解促进了黏附分子和趋化因子等的表达,招募中性粒细胞聚集于脑组织的血管内皮,促炎性细胞因子分泌可促进单核巨噬细胞等免疫炎症细胞的浸润和活化,同时小胶质细胞向促炎表型转化,后期T淋巴细胞浸润至脑实质,通过识别受损神经元暴露的抗原激活适应性免疫反应[5]。心脏骤停ROSC后先天性和适应性免疫反应均参与PCABI的进展,脑组织受缺血-再灌注打击受损后,早期活化的免疫细胞可分泌促炎性细胞因子等进一步激活其他类型的免疫细胞,逐步推动PCABI的发生发展。PCABI早期病理生理过程简易示意图如图 1所示。
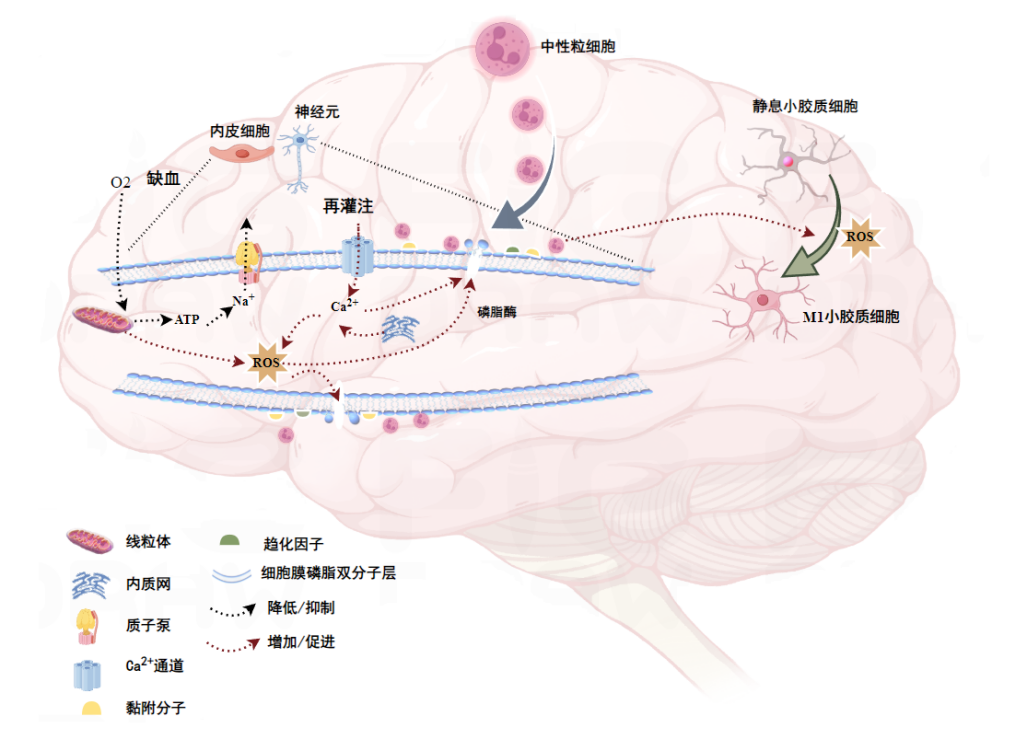
|
| 图 1 PCABI缺血-再灌注病理生理过程 |
|
|
血脑屏障(blood-brain barrier, BBB)是脑实质与外周循环的保护性屏障,由内皮细胞、周细胞、平滑肌细胞、星形胶质细胞和巨噬细胞共同组成,BBB的完整性对保护脑组织至关重要。研究发现心脏骤停后BBB的完整性受损[6],尽管及时有效地恢复自主循环,但是由于个体的差异性,部分ROSC后的患者BBB损伤仍持续进展[7],临床研究提示心脏骤停幸存者脑功能预后与BBB破坏程度相关[8]。
心脏骤停ROSC后BBB的损伤原因由多种原因引起,其主要病理特征为紧密连接的破坏。心脏骤停ROSC后由于脑内发生缺血-再灌注损伤后趋化因子等表达增加,率先向BBB浸润的为中性粒细胞,促炎型中性粒细胞(N1型)占据主导地位[9],其参与生成大量的活性氧(reactive oxygen species, ROS)和基质金属蛋白酶(Matrix metalloproteinase 9, MMP9)等,可直接损伤BBB的内皮细胞,从而破坏紧密连接[10]。随后其他免疫细胞如单核细胞和T淋巴细胞等迁移至BBB,共同参与BBB的破坏或修复。近期研究发现,在心脏骤停复苏后小鼠模型中,抑制γδ T细胞活化能够缓解BBB的损伤和大脑氧化应激[11],表明γδ T细胞可能参与心脏骤停后BBB损伤。研究表明脑组织缺氧后小胶质细胞活化[12],活化后的促炎性小胶质细胞衍生的外泌体能够介导BBB的损伤[13],此外小胶质细胞和中性粒细胞之间能够形成细胞通讯,互作参与BBB损伤[14]。心脏骤停ROSC后期BBB进入修复阶段,此阶段抑制性免疫细胞占主要地位参与调控其修复。研究发现心脏骤停ROSC后48 h观察到BBB开始修复[15],此时免疫抑制性细胞数量上调,例如N2表型中性粒细胞促进巨噬细胞吞噬促炎性中性粒细胞[9],M2型小胶质细胞上调紧密连接蛋白等表达来促进BBB修复,星形胶质细胞衍生的神经营养因子通过激活抗炎通路促进BBB的修复[16]。
心脏骤停后BBB的损伤是导致PCABI的关键机制之一,心脏骤停ROSC后早期阶段BBB完整性受损,初期主要由中性粒细胞和小胶质细胞介导免疫炎症损伤,其通透性的增加加重了外周循环免疫细胞进入脑实质的能力,进一步扩大脑内炎症反应,而后期抑制性免疫细胞协助修复受损的BBB。
3 免疫调控的神经元损伤 3.1 免疫器官免疫器官作为免疫细胞产生和分化发育的重要场所,心脏骤停ROSC后可能参与了体内免疫稳态失衡与PCABI进展相关。然而目前还未有研究详细表征人心脏骤停ROSC后中枢和外周免疫器官发生的形态和功能变化,以及和神经功能损伤之间的相关性。但是在动物模型中发现,窒息性心脏骤停ROSC后小鼠在亚急性期表现为脾脏和胸腺急剧萎缩,伴有胸腺和骨髓中T和B淋巴细胞生成受损[17]。然而在猪心脏骤停复苏的模型中观察到,早期体内免疫炎症反应激活时时脾脏无显著变化[18],造成此差异性结果的原因不明。在缺血-再灌注性脑损伤中研究发现,脑膜淋巴管在神经炎症级联反应中起重要作用,将脑内抗原等引流到颈部淋巴结来促进免疫反应[19]。近期Sun等[20]利用纳米探针示踪技术发现脑缺血-再灌注后脑内淋巴引流系统受损,主要表现淋巴内流受损和外流减少,阻碍了代谢废物的清除,可能加重免疫炎症反应。
免疫器官与神经系统的损伤相互交织,尽管脑内因缺血-再灌注激活了免疫反应,后因级联效应进一步扩大炎症反应,但是外周免疫器官也受到缺血-再灌注损伤造成其功能损伤,增加了继发性感染可能,而继发感染也可使脑损伤加重。
3.2 免疫细胞 3.2.1 中性粒细胞目前研究认为中性粒细胞是心脏骤停ROSC后早期阶段介导PCABI发生发展的免疫细胞。心脏骤停患者在ROSC后ICU入院时循环中性粒细胞来源的髓过氧化物酶(myeloperoxidase, MPO)水平升高,并且与持续脑电图异常相关[21],已知MPO与氧化应激性炎症损伤密切相关。还有研究表明心脏骤停复苏后12 h内血浆瓜氨酸组蛋白3(citrullinatedhistone H3, CitH3)升高,并与30 d神经功能预后不良相关[22]。CitH3是中性粒细胞胞外诱捕网(neutrophil extracellular traps, NETs)形成的生物标志物,NETs是中性粒细胞通过释放染色质和颗粒蛋白形成的网状结构,近期研究提示脑组织缺血后NETs能够通过激活炎症小体等途径介导神经元的焦亡[23-24]。脑内浸润的中性粒细胞分泌的CC基序趋化因子配体23(C-C motif chemokine ligand 23,CCL23)还能进一步趋化循环中性粒细胞向大脑募集,从而形成恶性循环,进一步促进局部脑组织炎症[25]。因此,心脏骤停ROSC后早期阶段中性粒细胞通过衍生的MPO等介导的神经组织损伤,以及通过趋化作用招募其他免疫细胞入脑等可能与PCABI进展相关。
3.2.2 T淋巴细胞目前T淋巴细胞与PCABI进展的相关性研究较少。有研究发现心脏骤停ROSC后脑组织内CD8+T细胞浸润[26],且伴随复苏时间的推移,CD8+T细胞数量呈上调趋势。高迁移率族蛋白B1(high mobility group box-1 protein,HMGB1)信号通路的激活可能与CD8+T细胞募集于脑组织有关[27]。在缺血性脑卒中的研究表明,小胶质细胞与大脑CD8+T细胞募集有关,并且CD8+T细胞的浸润加重了神经功能缺陷[28],在炎症性脑病中也发现CD8+T细胞能够与神经元互作,通过分泌干扰素-γ(interferon-γ, IFN-γ)和肿瘤坏死因子-α(tumor necrosis factor- α, TNF-α)介导皮质神经元损伤[29]。表明心脏骤停ROSC后脑内CD8+T的浸润可能与PCABI相关,未来需要进一步分析其亚群在脑缺血-再灌注不同阶段的动态变化,并研究CD8+T细胞在PCABI进展中的作用机制。
3.2.3 单核细胞/巨噬细胞目前研究提示外周来源的单核细胞/巨噬细胞与PCABI进展相关。近期,Thorp等[30]研究发现突发心血管事件后脑白质内单核细胞聚集且与神经功能损伤相关。根据细胞表面分子CD14和CD16的表达将单核细胞分为经典型(CD14++/CD16-)、中间型(CD14+/CD16+)和非经典型(CD14-/CD16++)三种类型,其中经典单核细胞表现出的强大的免疫促炎功能[31]。研究表明心脏骤停ROSC后患者体内经典型单核细胞数量上调[32],在小鼠脑组织缺氧后发现脑内Ly-6chigh单核细胞比例增加[33],小鼠Ly-6chigh单核细胞与人经典单核细胞相似,具有促炎功能,提示缺血-再灌注后经典型单核细胞占据主要地位可能与脑内炎症反应相关。在脑组织缺血性炎症中,单核细胞可以向促炎性巨噬细胞分化,Yang等[34]研究表明脑缺氧后促炎性巨噬细胞富集于脑组织,此类细胞高表达白细胞介素-1b、选择素L等基因,能编码白细胞介素-1β(interleukin-1β, IL-1β)、选择素L等,与促进免疫炎症反应相关。近期Chang等[35]研究提示,心脏骤停后肠道菌群失调,通过细菌来源的脂多糖(lipopolysaccharide, LPS)激活Toll样受体4(toll-like receptor4, TLR4),从而促进肠道内高表达髓细胞触发受体1(rriggering receptor expressed on myeloid cells-1, TREM1)的巨噬细胞向脑组织迁移,TREM1激活后会启动下游促炎细胞因子和趋化因子的产生的信号通路,从而发挥炎症放大作用[36]。还有研究发现[37],在心脏骤停患者复苏早期阶段,粘连蛋白细胞黏附分子2(Nectin-2)基因过表达的单核细胞亚群与神经功能预后不良有关,但是其参与的脑内炎症调控机制尚不明确。
3.2.4 调节性T细胞(regulatory cells, Treg)调节性免疫细胞可能在心脏骤停复苏后对神经功能起到保护和修复作用。在心脏骤停ROSC后的动物模型中观察到循环免疫细胞中Treg数量升高并与神经元功能预后相关[38],Gu等[39]研究表明ROSC后脾脏来源的Treg凋亡率降低。但是也有学者发现在ROSC后24 h循环Treg比例降低,72 h后幸存者Treg比例升高,Treg比例较高的患者生存率较高[40],表明Treg可能对脑功能具有保护作用。Treg在缺血性脑损伤后具有促进脑组织修复的功能,Treg能够直接通过分泌抗炎因子白细胞介素-10(interleukin-10, IL-10)等抑制神经炎症[41],此外还可与胶质细胞互作促进脑组织修复,例如Treg来源的骨桥蛋白通过小胶质细胞整合素受体增强小胶质细胞修复活性,促进少突形成和白质修复[42],Treg在缺血后脑组织富集并通过产生双调蛋白抑制神经毒性星形胶质细胞增殖来促进神经修复[43]。Treg通过抑制炎症、调节免疫和促进修复在PCABI进展后期具有重要作用。
3.2.5 小胶质细胞小胶质细胞作为神经系统固有免疫细胞,在PCABI进展过程中展现双重作用。生理条件下小胶质细胞通常处于静息状态,某些病理条件下能够向经典激活型小胶质细胞(M1型)转化。大量研究表明心脏骤停ROSC后早期小胶质细胞向M1型极化[44-48],丘脑网状核、海马CA1区域和齿状回等区域均发现了小胶质细胞富集和活化,同时观察到相应部位神经元损伤[44-45]。多种途径诱导心脏骤停ROSC后小胶质细胞极化,脑缺血-再灌注后趋化因子受体CX3CL1/CX3CR1轴过度激活可诱导小胶质细胞向M1型转化[46],脑组织缺氧下脑微血管内皮细胞衍生的外泌体可能协助小胶质细胞向促炎表型转化[47],心脏骤停复苏后肥大细胞类胰蛋白酶上调通过激活蛋白酶活化受体2(protease-activated receptor 2, PAR2)/P38/核转录因子-κB(nuclear factor kappa B, NF-κB)信号通路促进小胶质细胞促炎表型,介导海马体神经元变性[49]。此外研究表明脑内其他免疫细胞分泌的IFN-γ,以及细胞损伤后产生的ROS、MMP和LPS等均可促进小胶质细胞向M1型转化[50]。M1型小胶质细胞介导神经元和脑血管损伤,心脏骤停后M1型小胶质细胞的TLR4/TLR髓样分化因子88(myeloid differentiation factor 88, MyD88)/NF-κB信号通路过度激活[51],NF-κB通路激活后参与促炎因子TNF-α、白细胞介素-6(interleukin-6,IL-6)等分泌[52-53]。此外,ROSC后小胶质细胞焦亡与神经元损伤密切相关,M1型小胶质细胞通过激活胞内核苷酸结合寡聚结构域样受体蛋白3(nucleotide-binding oligomerization domain-like receptor protein 3, NLRP3)炎症小体细胞自身焦亡,此过程中细胞内大量的促炎物质释放,从而引起强烈的神经元炎症性损伤[48]。LPS激活的小胶质细胞增加了MMP9释放[54],MMP9在PCABI中与脑血管破坏和脑水肿发生有关[5]。在ROSC后数天至数周,部分小胶质细胞转向替代激活型(M2型),发挥抗炎和修复作用,能够通过胞吞清除凋亡细胞并重塑脑内炎症微环境[55]。表达趋化因子配体2(C-C motif chemokine ligand 2,CCL2)和趋化因子配体8(CCL8)的小胶质细胞还能通过趋化因子受体2(CCR2)/趋化因子受体5(CCR5)来促进CD8+T细胞进入脑组织介导适应性免疫损伤[56]。
综上,心脏骤停ROSC后免疫细胞与PCABI的关系呈现高度复杂性,目前研究表明与PCABI进展密切相关的促炎免疫细胞包括中性粒细胞、促炎性单核细胞和M1型小胶质细胞,通过分泌细胞因子等途径介导神经元损伤,CD8+T细胞可能也参与神经元损伤,但是具体机制仍需进一步研究,Treg和M2型小胶质细胞具有免疫调节并参与修复受损神经元。未来研究应进一步了解PCABI进展过程中免疫细胞动态变化谱,以及其他免疫细胞如CD4+T细胞、B淋巴细胞等在PCABI中扮演的角色。
3.3 细胞因子PCABI进展中由免疫细胞分泌的促炎细胞因子是介导神经元直接损伤的重要免疫分子,其中目前研究较为广泛且与脑损伤密切相关的促炎因子包括TNF-α、IL-6和IL-1家族。临床研究表明,心脏骤停ROSC后患者体内部分细胞因子水平发生波动,其中循环IL-6水平显著升高[57-58],且与患者预后呈负相关[59],神经功能预后良好的患者血清IL-6和TNF-α水平维持较低趋势[60],表明TNF-α和IL-6参与了脑功能的破坏。在动物心脏骤停模型中,大脑皮质、海马体和纹状体中TNF-α、IL-6和IL-1α水平显著升高,同时也发现免疫抑制性因子IL-10水平升高[61]。IL-6由单核细胞和T淋巴细胞等细胞分泌,能够激活小胶质细胞和星形胶质细胞向促炎表型转化促进PCABI的进展[62]。有研究表明大脑经历缺血-再灌注后,IL-6通过激活Janus激酶/信号传导及转录激活蛋白3(signal transducer and activator of transcription 3, STAT3)信号轴加剧了星形胶质细胞的炎症反应[63],IL-6/STAT3轴激活通过下调激活转录因子6(activating transcription factor, ATF6)表达诱导缺氧神经元凋亡[64],研究证实抑制或中和IL-6有利于心脏骤停复苏后脑神经功能的恢复[65-66]。TNF-α主要引起引起神经元凋亡和导致脑血管内皮受损,脑内TNF-α可诱导的海马神经元坏死性凋亡[67-68]。研究发现TNF-α通过激活STAT3通路促进星形胶质细胞向炎症反应表型转变,导致BBB功能障碍[69],TNF-α还通过激活受体相互作用蛋白(receptor interacting protein-1, RIP1)/RIP3/蛋白激酶B(protein kinase B, PKB)信号通路促进脑缺血后血小板聚集,引起微循环障碍加重脑损伤[70],TNF-α还能通过TNF受体促进脑微血管内皮细胞MMP9产生介导血管损伤[71]。然而脑缺血缺氧后局部乳酸浓度升高,乳酸通过泛素化稳定星形胶质细胞的N-myc下游调节基因2(N-myc downstream regulated gene 2, NDRG2)从而抑制TNF-α的表达,进而抑制反应性星形胶质细胞的形成协助缺氧性脑神经功能的恢复[72]。之前所述小胶质细胞在心脏骤停后NLRP3激活,小胶质细胞的NLRP3激活与其对IL-1β的成熟和释放相关[73],反之小胶质细胞释放的IL-1β又通过IL-1β/IL-1R1/TRAF6信号轴激活神经元和内皮细胞的NLRP3炎性小体,介导神经元焦亡和促进免疫细胞脑浸润[74]。此外,近几年促炎因子HMGB1在心脏骤停后神经促炎作用也受到关注,研究发现心脏骤停ROSC后1周内患者血清HMGB1水平升高,急性生理与慢性健康评分(APACHE Ⅱ评分)和HMGB1水平呈正相关[75],HMGB1激活TLR4/NF-κB信号通路[76],促进小胶质细胞促炎因子释放,此外HMGB1还能促进心脏骤停后CD8+T向脑组织浸润[77]。
综上,心脏骤停ROSC后细胞因子在PCABI中起核心作用,TNF-α、IL-6在急性期可直接引起神经元和血管损伤,还参与激活其他免疫细胞,后期免疫抑制性细胞因子IL-10可能占据主导地位支持修复,目前需要更多的临床研究进一步探索各细胞因子在心脏骤停ROSC后不同时期的变化趋势以及和脑功能预后的关系。
3.4 补体系统补体是调节免疫反应的重要蛋白,研究发现心脏骤停ROSC后患者血清中补体活化产物C3bc、可溶性末端补体复合物sC5b-9水平升高,并且与脑功能的不良预后相关[78],Wang等[79]研究发现血清可溶性CD59(sCD59)水平升高并与心脏骤停ROSC后神经损伤的严重程度呈正相关,可用于预测神经功能的预后。CD59可存在于神经元细胞膜中,血清sCD59水平升高可能预示神经元的破坏,但不能排除其他来源。现有研究仅提示异常表达的补体可能参与PCABI的进展,然而其调控机制尚未完全阐明,有研究表明缺血-再灌注后所形成的补体复合物协助激活免疫细胞[80],因此补体可能通过激活其他促炎免疫细胞与神经功能损伤相关。
3.5 炎症小体(Inflammasome)目前研究发现与PCABI进展有关的炎症小体包括NLR家族的NLRP3炎症小体和PYHIN家族的黑色素瘤缺乏因子2(absent in melanoma 2, AIM2)炎症小体。NLRP3炎症小体是一种多蛋白复合物,在调节先天免疫反应和炎症信号转导中发挥着重要功能,研究发现心脏骤停ROSC后脑内NLRP3炎症小体上调与PCABI的进展密切相关[81-83],心脏骤停ROSC后和缺血性脑卒中均发现NLRP3炎症小体的激活,并通过启动小胶质细胞和星形胶质细胞焦亡途径加重脑组织免疫炎症性损伤[48, 84-85],Pan等[74]研究提示脑缺血-再灌注后小胶质细胞分泌的IL-1β能够激活神经元和内皮细胞的NLRP3炎症小体,促进神经元焦亡和血管损伤。心脏骤停复苏后NLRP3通过多种途径激活,例如小胶质细胞钾离子外流[82],IL-1受体/TRAF6依赖性途径[74]和HMGB1/TLR4/NLRP3通路[86]等。当选择性抑制NLRP3炎症小体后,心脏骤停复苏后大鼠的血清IL-1β和神经元特异性烯醇化酶(Neuron specific enolase, NSE)水平降低,并表现出神经功能的改善[87],表明心脏骤停复苏后NLRP3炎症小体通过介导神经元焦亡和促炎细胞因子IL-1β的分泌参与PCABI进展。AIM2是组成炎症小体重要的模式识别受体之一,在固有免疫应答中发挥着重要作用,近期研究表明AIM2炎性小体激活同样通过诱导神经元焦亡与缺血-再灌注后脑损伤有关[88],Shao等[89]研究表明大鼠心脏骤停ROSC后伴随神经元AIM2炎性小体激活,AIM2激活后通过诱导神经元焦亡和抑制带电多泡体蛋白2A(charged multivesicular body protein 2A, CHMP2A)介导的细胞自噬增强神经元炎症反应。心脏骤停ROSC后脑内NF-κB信号通路激活[49],NF-κB信号通路激活与AIM2炎症小体的活化有关[90]。此外研究还发现脑组织缺血缺氧后内皮细胞的AIM2上调并促进BBB的中性粒细胞黏附和紧密连接蛋白降解,增加BBB通透性[91]。因此,NLRP3炎症小体和AIM2炎性小体在心脏骤停后神经损伤中通过释放IL-1β、诱导焦亡和破坏自噬加重脑损伤,未来在靶向炎症小体治疗方面需结合时间窗特异性干预(如急性期抑制NLRP3,恢复期增强自噬)。
4 免疫调节与PCABI的治疗 4.1 临床研究低温治疗作为脑保护的重要治疗措施临床中早已应用于心脏骤停复苏后昏迷患者,其治疗原理包括减少氧自由基,减少损伤后炎症反应。近几年陆续开展了细胞因子靶向治疗的临床研究,前期一项研究表明[66],IL-6受体拮抗剂托珠单抗治疗减轻了院外心脏骤停后全身炎症反应,但是患者神经系统结局无显著变化。近期一项细胞因子吸附治疗的相关研究表明,细胞因子血液吸附治疗能够有效降低血液中IL-6水平,且其安全性得到认可,但是该研究未进一步探索该治疗手段对神经系统结局的影响[92]。STEROHCA的一项亚研究试验(NCT04624776)表明[93],院外心脏骤停复苏患者院前应用大剂量糖皮质激素干预24小时后,抗炎细胞因子IL-10增加而促炎细胞因子IL-6、IL-8和TNF-α水平降低,患者180 d生存率较安慰剂组升高了11%,表明下调复苏早期的促炎细胞因子可能有助于提高患者生存率。
4.2 基础研究在动物心脏骤停模型中发现,抑制促炎反应相关通路有助于改善神经功能。例如通过抑制NLRP3炎症小体、AIM2炎症小体和HMGB1炎症因子相关促炎信号通路可以降低促炎细胞因子产生等,从而减轻脑内免疫炎症反应以保护神经功能[27, 94-95],超快速低体温治疗可以减轻心脏骤停复苏后早期阶段的体液免疫反应来保护神经功能[79]。调节神经元线粒体可能改善心脏骤停后脑损伤[96],近两年研究表明,健康的线粒体能够缓解组织细胞缺血-再灌注后过度的氧化应激,减少促炎因子等分泌[97],Hayashida等[98]研究显示,将新鲜线粒体移植入心脏骤停复苏后动物神经元内能够帮助恢复脑微循环并改善神经功能。线粒体移植作为帮助细胞恢复功能的治疗手段,还可能通过ROS清除途径在一定程度降低了由ROS引起的促炎信号通路的激活。由于神经系统受损后修复能力较差,研究发现心脏骤停后脑室内给予神经干细胞能够改善复苏后的神经功能[99],近期研究提示神经干细胞移植入心脏骤停大鼠脑内还能够抑制NLRP3炎症小体活化和小胶质细胞的促炎表型转化,从而缓解PCABI的进展[100]。
综上所述,免疫调节在PCABI的治疗中仍具有重要潜力,尽管目前现有的临床研究结果对PCABI的预后未展现出显著的积极影响,但越来越多的临床前研究表明早期调控过度的促炎反应对神经元的保护的有效的。调节免疫的优点在于一定程度上控制强烈的免疫反应引起的神经元和BBB的损伤,控制炎症级联反应造成的神经功能不良预后,但是在临床实践中应考虑治疗的时间窗和患者个体差异性,需要根据患者免疫激活-修复阶段的动态变化指标指导治疗,避免不适时过度抑制免疫造成“细菌异位”等其他继发性感染,需在抑制过度炎症与保留修复功能间取得平衡。此外还应考虑如用免疫调节类药物时药物的BBB穿透率。
5 总结与展望PCABI的核心病理过程是心脏骤停后的全身缺血-再灌注损伤,引发脑组织免疫炎症反应激活导致神经元死亡和神经功能缺损。PCABI发生发展由复杂的免疫网络调控,缺血-再灌注引起的趋化因子和黏附分子等表达的变化,促进中性粒细胞向脑组织浸润,破坏BBB和神经元,伴随小胶质细胞M1极化,随后外周免疫细胞浸润脑组织,分泌促炎因子并激活补体、相关炎症小体,诱导神经元焦亡。后期Treg、M2型小胶质细胞等免疫调节细胞参与受损神经元修复。现阶段研究的局限性与未来机遇:(1)PCABI进展过程中多种免疫细胞均有参与,但是各细胞组分间随复苏后时间推移的动态变化谱尚未完全阐明,未来还应进一步明确免疫激活和免疫修复的时间窗,以预测损伤和指导治疗;(2)近几年基础研究中的动物模型多以未经免疫系统人源化重建的啮齿类动物为主,可能无法更贴切的模拟人类PCABI的免疫微环境;(3)在临床研究中,目前还缺乏预测神经功能预后的可靠免疫学指标;(4)目前临床前研究与临床研究存在一定的鸿沟,例如大量基础研究支持IL-6在PCABI进展早期对神经元的损伤作用,然而靶向IL-6未观察到有效的脑保护作用,基于PCABI免疫微环境的复杂性,可以进行多模态联合治疗的探索,后期还可以根据免疫指标与神经功能预后的相关性,进一步探索其他免疫靶点的治疗效果;(5)由于BBB药物穿透性难题,未来或可考虑构建靶向免疫因子药物的工程化递送载体和仿生策略等;(6)PCABI的进展和治疗结果均具有一定的个体差异性,但现有研究多基于群体水平,因此还应考虑个体差异如促炎因子的基因多态性引起不同的免疫反应强度,治疗时也应考虑患者免疫损伤-修复的动态时间窗,以实现精准化个体治疗。最后,期待未来的研究使得PCABI发生发展的机制得以进一步阐明并在治疗方面取得新的突破。
利益冲突 所有作者声明无利益冲突
| [1] | 兰超, 张强, 雷如意, 等. 心脏骤停救治现状及2023年研究热点[J]. 中华急诊医学杂志, 2024, 33(1): 6-10. DOI:10.3760/cma.j.issn.1671-0282.2024.01.002 |
| [2] | 中国心脏骤停与心肺复苏报告编写组, 徐峰, 陈玉国. 中国心脏骤停与心肺复苏报告(2022年版)概要[J]. 中国循环杂志, 2023, 38(10): 1005-1017. DOI:10.3969/j.issn.1000-3614.2023.10.002 |
| [3] | Sekhon MS, Ainslie PN, Griesdale DE. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a "two-hit" model[J]. Crit Care, 2017, 21(1): 90. DOI:10.1186/s13054-017-1670-9 |
| [4] | Douglas-Escobar M, Weiss MD. Hypoxic-ischemic encephalopathy: a review for the clinician[J]. JAMA Pediatr, 2015, 169(4): 397-403. DOI:10.1001/jamapediatrics.2014.3269 |
| [5] | Cai SQ, Li Q, Fan JJ, et al. Therapeutic hypothermia combined with hydrogen sulfide treatment attenuated early blood-brain barrier disruption and brain edema induced by cardiac arrest and resuscitation in rat model[J]. Neurochem Res, 2023, 48(3): 967-979. DOI:10.1007/s11064-022-03824-5 |
| [6] | Jiang TX, Li YN, Liu HH, et al. Blood-brain barrier disruption and neuroinflammation in the hippocampus of a cardiac arrest porcine model: Single-cell RNA sequencing analysis[J]. Neural Regen Res, 2026, 21(2): 742-755. DOI:10.4103/NRR.NRR-D-24-01269 |
| [7] | You Y, Park JS, Min JH, et al. Blood-brain barrier permeability for the first 24 hours in hypoxic-ischemic brain injury following cardiac arrest[J]. Resuscitation, 2024, 198: 110150. DOI:10.1016/j.resuscitation.2024.110150 |
| [8] | Kang C, You Y, Ahn HJ, et al. Blood-brain barrier disruption as a cause of various serum neuron-specific enolase cut-off values for neurological prognosis in cardiac arrest patients[J]. Sci Rep, 2022, 12(1): 2186. DOI:10.1038/s41598-022-06233-4 |
| [9] | Xie MZ, Hao YL, Feng LS, et al. Neutrophil heterogeneity and its roles in the inflammatory network after ischemic stroke[J]. Curr Neuropharmacol, 2023, 21(3): 621-650. DOI:10.2174/1570159X20666220706115957 |
| [10] | Qiu YM, Zhang CL, Chen AQ, et al. Immune cells in the BBB disruption after acute ischemic stroke: targets for immune therapy?[J]. Front Immunol, 2021, 12: 678744. DOI:10.3389/fimmu.2021.678744 |
| [11] | Li YQ, Wu T, Guo C. Inhibition of γδ T cells alleviates blood-brain barrier in cardiac arrest and cardiopulmonary resuscitation in mice[J]. Mol Biotechnol, 2023, 65(12): 2061-2070. DOI:10.1007/s12033-023-00705-2 |
| [12] | Ling Y, Jin L, Ma QX, et al. Salvianolic acid A alleviated inflammatory response mediated by microglia through inhibiting the activation of TLR2/4 in acute cerebral ischemia-reperfusion[J]. Phytomedicine, 2021, 87: 153569. DOI:10.1016/j.phymed.2021.153569 |
| [13] | Jiang W, Wu Y, Pang AL, et al. M1-type microglia-derived exosomes contribute to blood-brain barrier damage[J]. Brain Res, 2024, 1835: 148919. DOI:10.1016/j.brainres.2024.148919 |
| [14] | Yoon SH, Kim CY, Lee E, et al. Microglial NLRP3-gasdermin D activation impairs blood-brain barrier integrity through interleukin-1β-independent neutrophil chemotaxis upon peripheral inflammation in mice[J]. Nat Commun, 2025, 16(1): 699. DOI:10.1038/s41467-025-56097-1 |
| [15] | Zhang C, Brandon NR, Koper K, et al. Invasion of peripheral immune cells into brain parenchyma after cardiac arrest and resuscitation[J]. Aging Dis, 2018, 9(3): 412-425. DOI:10.14336/AD.2017.0926 |
| [16] | Han D, Li FY, Zhang HX, et al. Mesencephalic astrocyte-derived neurotrophic factor restores blood-brain barrier integrity of aged mice after ischaemic stroke/reperfusion through anti-inflammation via TLR4/MyD88/NF-κB pathway[J]. J Drug Target, 2022, 30(4): 430-441. DOI:10.1080/1061186X.2021.2003803 |
| [17] | Wang W, Li R, Miao WY, et al. Development and evaluation of a novel mouse model of asphyxial cardiac arrest revealed severely impaired lymphopoiesis after resuscitation[J]. J Am Heart Assoc, 2021, 10(11): e019142.. DOI:10.1161/JAHA.120.019142 |
| [18] | Rolland TJ, Hudson ER, Graser LA, et al. Splenic modulation of the early inflammatory response to regional and global ischemia/reperfusion injury in swine[J]. Am J Physiol Heart Circ Physiol, 2025. DOI:10.1152/ajpheart.00714.2024 |
| [19] | Machado RS, Mathias K, Joaquim L, et al. Emerging roles of meningeal lymphatic vessels in ischemic stroke[J]. Mol Neurobiol, 2025. DOI:10.1007/s12035-025-04983-6 |
| [20] | Sun B, Fang DL, Li WZ, et al. NIR-II nanoprobes for investigating the glymphatic system function under anesthesia and stroke injury[J]. J Nanobiotechnology, 2024, 22(1): 200. DOI:10.1186/s12951-024-02481-w |
| [21] | Pekkarinen PT, Carbone F, Minetti S, et al. Markers of neutrophil mediated inflammation associate with disturbed continuous electroencephalogram after out of hospital cardiac arrest[J]. Acta Anaesthesiol Scand, 2023, 67(1): 94-103. DOI:10.1111/aas.14145 |
| [22] | Mauracher LM, Buchtele N, Schörgenhofer C, et al. Increased citrullinated histone H3 levels in the early post-resuscitative period are associated with poor neurologic function in cardiac arrest survivors-a prospective observational study[J]. J Clin Med, 2019, 8(10): 1568. DOI:10.3390/jcm8101568 |
| [23] | Cao YY, Shi MM, Liu L, et al. Inhibition of neutrophil extracellular trap formation attenuates NLRP1-dependent neuronal pyroptosis via STING/IRE1α pathway after traumatic brain injury in mice[J]. Front Immunol, 2023, 14: 1125759. DOI:10.3389/fimmu.2023.1125759 |
| [24] | Tang CM, Jia F, Wu M, et al. Elastase-targeting biomimic nanoplatform for neurovascular remodeling by inhibiting NETosis mediated AlM2 inflammasome activation in ischemic stroke[J]. J Control Release, 2024, 375: 404-421. DOI:10.1016/j.jconrel.2024.09.026 |
| [25] | DeKay JT, Chepurko E, Chepurko V, et al. Delayed CCL23 response is associated with poor outcomes after cardiac arrest[J]. Cytokine, 2024, 176: 156536. DOI:10.1016/j.cyto.2024.156536 |
| [26] | Smida T, Koller AC, Menegazzi JJ, et al. Early cytotoxic lymphocyte localization to the brain following resuscitation in a porcine model of asphyxial cardiac arrest: a pilot study[J]. Resusc Plus, 2021, 6: 100125. DOI:10.1016/j.resplu.2021.100125 |
| [27] | Boissady E, Abi Zeid Daou Y, Faucher E, et al. High-mobility group box 1-signaling inhibition with glycyrrhizin prevents cerebral T-cell infiltration after cardiac arrest[J]. J Am Heart Assoc, 2023, 12(3): e027749.. DOI:10.1161/JAHA.122.027749 |
| [28] | Ma YL, Zheng K, Zhao CC, et al. Microglia LILRB4 upregulation reduces brain damage after acute ischemic stroke by limiting CD8+ T cell recruitment[J]. J Neuroinflammation, 2024, 21(1): 214. DOI:10.1186/s12974-024-03206-4 |
| [29] | Lin CH, Scheller A, Liu Y, et al. Study of effector CD8+ T cell interactions with cortical neurons in response to inflammation in mouse brain slices and neuronal cultures[J]. Int J Mol Sci, 2023, 24(4): 3166. DOI:10.3390/ijms24043166 |
| [30] | Thorp EB, Filipp M, Dima M, et al. CCR2+ monocytes promote white matter injury and cognitive dysfunction after myocardial infarction[J]. Brain Behav Immun, 2024, 119: 818-835. DOI:10.1016/j.bbi.2024.05.004 |
| [31] | Cormican S, Negi N, Naicker SD, et al. Chronic kidney disease is characterized by expansion of a distinct proinflammatory intermediate monocyte subtype and by increased monocyte adhesion to endothelial cells[J]. J Am Soc Nephrol, 2023, 34(5): 793-808. DOI:10.1681/ASN.0000000000000083 |
| [32] | Asmussen A, Busch HJ, Helbing T, et al. Monocyte subset distribution and surface expression of HLA-DR and CD14 in patients after cardiopulmonary resuscitation[J]. Sci Rep, 2021, 11(1): 12403. DOI:10.1038/s41598-021-91948-z |
| [33] | Wicks EE, Ran KR, Kim JE, et al. The translational potential of microglia and monocyte-derived macrophages in ischemic stroke[J]. Front Immunol, 2022, 13: 897022. DOI:10.3389/fimmu.2022.897022 |
| [34] | Yang M, Li YX, Shi KB, et al. Single-cell transcriptomes of immune cells from multiple compartments redefine the ontology of myeloid subtypes post-stroke[J]. Adv Sci (Weinh), 2025, 12(13): e2408722.. DOI:10.1002/advs.202408722 |
| [35] | Chang Y, Chen JC, Peng YQ, et al. Gut-derived macrophages link intestinal damage to brain injury after cardiac arrest through TREM1 signaling[J]. Cell Mol Immunol, 2025, 22(4): 437-455. DOI:10.1038/s41423-025-01263-0 |
| [36] | Li CY, Cai CJ, Xu DF, et al. TREM1:Activation, signaling, cancer and therapy[J]. Pharmacol Res, 2024, 204: 107212. DOI:10.1016/j.phrs.2024.107212 |
| [37] | Tamura T, Cheng CD, Chen WA, et al. Single-cell transcriptomics reveal a hyperacute cytokine and immune checkpoint axis after cardiac arrest in patients with poor neurological outcome[J]. Med, 2023, 4(7): 432-456. DOI:10.1016/j.medj.2023.05.003 |
| [38] | Dou HY, Brandon NR, Koper KE, et al. Fingerprint of circulating immunocytes as biomarkers for the prognosis of brain inflammation and neuronal injury after cardiac arrest[J]. ACS Chem Neurosci, 2023, 14(23): 4115-4127. DOI:10.1021/acschemneuro.3c00397 |
| [39] | Gu W, Zhang Q, Li CS. Effect of splenic regulatory T-cell apoptosis on the postresuscitation immune dysfunction in a porcine model[J]. Chin Med J (Engl), 2016, 129(13): 1577-1583. DOI:10.4103/0366-6999.184461 |
| [40] | Qi ZJ, Liu Q, Zhang Q, et al. Overexpression of programmed cell death-1 and human leucocyte antigen-DR on circulatory regulatory T cells in out-of-hospital cardiac arrest patients in the early period after return of spontaneous circulation[J]. Resuscitation, 2018, 130: 13-20. DOI:10.1016/j.resuscitation.2018.06.023 |
| [41] | Zheng Y, Ren Z, Liu Y, et al. T cell interactions with microglia in immune-inflammatory processes of ischemic stroke[J]. Neural Regen Res, 2025, 20(5): 1277-1292. DOI:10.4103/nrr.nrr-d-23-01385 |
| [42] | Shi LG, Sun ZY, Su W, et al. Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke[J]. Immunity, 2021, 54(7): 1527-1542. DOI:10.1016/j.immuni.2021.04.022 |
| [43] | Ito M, Komai K, Mise-Omata S, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery[J]. Nature, 2019, 565(7738): 246-250. DOI:10.1038/s41586-018-0824-5 |
| [44] | Ton HT, Raffensperger K, Shoykhet M. Early thalamic injury after resuscitation from severe asphyxial cardiac arrest in developing rats[J]. Front Cell Dev Biol, 2021, 9: 737319. DOI:10.3389/fcell.2021.737319 |
| [45] | Ousta A, Piao L, Fang YH, et al. Microglial activation and neurological outcomes in a murine model of cardiac arrest[J]. Neurocrit Care, 2022, 36(1): 61-70. DOI:10.1007/s12028-021-01253-w |
| [46] | Mao M, Xu Y, Zhang XY, et al. microRNA-195 prevents hippocampal microglial/macrophage polarization towards the M1 phenotype induced by chronic brain hypoperfusion through regulating CX3CL1/CX3CR1 signaling[J]. J Neuroinflammation, 2020, 17(1): 244. DOI:10.1186/s12974-020-01919-w |
| [47] | Min XL, Jia WJ, Guo L, et al. Brain microvascular endothelial cell-derived exosomes transmitting circ_0000495 promote microglial M1-polarization and endothelial cell injury under hypoxia condition[J]. FASEB J, 2024, 38(2): e23387. DOI:10.1096/fj.202301637R |
| [48] | Chang Y, Zhu J, Wang D, et al. NLRP3 inflammasome-mediated microglial pyroptosis is critically involved in the development of post-cardiac arrest brain injury[J]. J Neuroinflammation, 2020, 17(1): 219. DOI:10.1186/s12974-020-01879-1 |
| [49] | Ocak U, Eser Ocak P, Huang L, et al. Inhibition of mast cell tryptase attenuates neuroinflammation via PAR-2/p38/NFκB pathway following asphyxial cardiac arrest in rats[J]. J Neuroinflammation, 2020, 17(1): 144. DOI:10.1186/s12974-020-01808-2 |
| [50] | Denver P, Cunningham C. Microglial activation and neuroinflammation in acute and chronic cognitive deficits in sepsis[J]. Neuropharmacology, 2025, 267: 110285. DOI:10.1016/j.neuropharm.2024.110285 |
| [51] | Sun JF, Lu LP, Lian YT, et al. Sodium butyrate attenuates microglia-mediated neuroinflammation by modulating the TLR4/MyD88/NF-κB pathway and microbiome-gut-brain axis in cardiac arrest mice[J]. Mol Brain, 2025, 18(1): 13. DOI:10.1186/s13041-025-01179-w |
| [52] | Xu S, Guo L, Shao WJ, et al. Vagus nerve stimulation protects against cerebral injury after cardiopulmonary resuscitation by inhibiting inflammation through the TLR4/NF-κB and α7nAChR/JAK2 signaling pathways[J]. World J Emerg Med, 2023, 14(6): 462-470. DOI:10.5847/wjem.j.1920-8642.2023.102 |
| [53] | Yang Y, Fei YX, Xu XJ, et al. Shikonin attenuates cerebral ischemia/reperfusion injury via inhibiting NOD2/RIP2/NF-κB-mediated microglia polarization and neuroinflammation[J]. J Stroke Cerebrovasc Dis, 2024, 33(6): 107689. DOI:10.1016/j.jstrokecerebrovasdis.2024.107689 |
| [54] | Vahsen BF, Nalluru S, Morgan GR, et al. C9orf72-ALS human iPSC microglia are pro-inflammatory and toxic to co-cultured motor neurons via MMP9[J]. Nat Commun, 2023, 14(1): 5898. DOI:10.1038/s41467-023-41603-0 |
| [55] | Zhang KX, Zhang YZ, Li ZT, et al. Potentiating microglial efferocytosis by MFG-E8 improves survival and neurological outcome after successful cardiopulmonary resuscitation in mice[J]. Brain Pathol, 2024: e13327. DOI:10.1111/bpa.13327 |
| [56] | Shi ZS, Yu P, Lin WJ, et al. Microglia drive transient insult-induced brain injury by chemotactic recruitment of CD8+ T lymphocytes[J]. Neuron, 2023, 111(5): 696-710. DOI:10.1016/j.neuron.2022.12.009 |
| [57] | Hoiland RL, Ainslie PN, Wellington CL, et al. Brain hypoxia is associated with neuroglial injury in humans post-cardiac arrest[J]. Circ Res, 2021, 129(5): 583-597. DOI:10.1161/CIRCRESAHA.121.319157 |
| [58] | Akin M, Sieweke JT, Garcheva V, et al. Additive impact of interleukin 6 and neuron specific enolase for prognosis in patients with out-of-hospital cardiac arrest-experience from the HAnnover COoling REgistry[J]. Front Cardiovasc Med, 2022, 9: 899583. DOI:10.3389/fcvm.2022.899583 |
| [59] | Langeland H, Damås JK, Mollnes TE, et al. The inflammatory response is related to circulatory failure after out-of-hospital cardiac arrest: a prospective cohort study[J]. Resuscitation, 2022, 170: 115-125. DOI:10.1016/j.resuscitation.2021.11.026 |
| [60] | Patel JK, Sinha N, Hou W, et al. Association of post-resuscitation inflammatory response with favorable neurologic outcomes in adults with in-hospital cardiac arrest[J]. Resuscitation, 2021, 159: 54-59. DOI:10.1016/j.resuscitation.2020.12.014 |
| [61] | Uray T, Dezfulian C, Palmer AA, et al. Cardiac arrest induced by asphyxia versus ventricular fibrillation elicits comparable early changes in cytokine levels in the rat brain, heart, and serum[J]. J Am Heart Assoc, 2021, 10(5): e018657. DOI:10.1161/JAHA.120.018657 |
| [62] | Kerkis I, da Silva áP, Araldi RP. The impact of interleukin-6 (IL-6) and mesenchymal stem cell-derived IL-6 on neurological conditions[J]. Front Immunol, 2024, 15: 1400533. DOI:10.3389/fimmu.2024.1400533 |
| [63] | Cheng M, Liang XS, Shi LR, et al. Folic acid deficiency exacerbates the inflammatory response of astrocytes after ischemia-reperfusion by enhancing the interaction between IL-6 and JAK-1/pSTAT3[J]. CNS Neurosci Ther, 2023, 29(6): 1537-1546. DOI:10.1111/cns.14116 |
| [64] | Zhou SM, Zhong ZF, Huang P, et al. IL-6/STAT3 induced neuron apoptosis in hypoxia by downregulating ATF6 expression[J]. Front Physiol, 2021, 12: 729925. DOI:10.3389/fphys.2021.729925 |
| [65] | Meyer MAS, Bjerre M, Wiberg S, et al. Modulation of inflammation by treatment with tocilizumab after out-of-hospital cardiac arrest and associations with clinical status, myocardial- and brain injury[J]. Resuscitation, 2023, 184: 109676. DOI:10.1016/j.resuscitation.2022.109676 |
| [66] | Meyer MAS, Wiberg S, Grand J, et al. Treatment effects of interleukin-6 receptor antibodies for modulating the systemic inflammatory response after out-of-hospital cardiac arrest (the IMICA trial): a double-blinded, placebo-controlled, single-center, randomized, clinical trial[J]. Circulation, 2021, 143(19): 1841-1851. DOI:10.1161/CIRCULATIONAHA.120.053318 |
| [67] | Wang WY, Xie L, Zou XS, et al. Pomelo peel oil suppresses TNF-α-induced necroptosis and cerebral ischaemia-reperfusion injury in a rat model of cardiac arrest[J]. Pharm Biol, 2021, 59(1): 401-409. DOI:10.1080/13880209.2021.1903046 |
| [68] | Wang L, Deng B, Yan PP, et al. Neuroprotective effect of ketamine against TNF-α-induced necroptosis in hippocampal neurons[J]. J Cell Mol Med, 2021, 25(7): 3449-3459. DOI:10.1111/jcmm.16426 |
| [69] | Kim H, Leng K, Park J, et al. Reactive astrocytes transduce inflammation in a blood-brain barrier model through a TNF-STAT3 signaling axis and secretion of alpha 1-antichymotrypsin[J]. Nat Commun, 2022, 13(1): 6581. DOI:10.1038/s41467-022-34412-4 |
| [70] | Li W, Liu DP, Xu JQ, et al. Astrocyte-derived TNF-α-activated platelets promote cerebral ischemia/reperfusion injury by regulating the RIP1/RIP3/AKT signaling pathway[J]. Mol Neurobiol, 2022, 59(9): 5734-5749. DOI:10.1007/s12035-022-02942-z |
| [71] | Lee TH, Chen JL, Tsai MM, et al. Protective effects of sophoraflavanone G by inhibiting TNF-α-induced MMP-9-mediated events in brain microvascular endothelial cells[J]. Int J Mol Sci, 2023, 25(1): 283. DOI:10.3390/ijms25010283 |
| [72] | Xu JY, Ji T, Li GC, et al. Lactate attenuates astrocytic inflammation by inhibiting ubiquitination and degradation of NDRG2 under oxygen-glucose deprivation conditions[J]. J Neuroinflammation, 2022, 19(1): 314. DOI:10.1186/s12974-022-02678-6 |
| [73] | Zeng ZJ, Lin XB, Yang L, et al. Activation of inflammasomes and relevant modulators for the treatment of microglia-mediated neuroinflammation in ischemic stroke[J]. Mol Neurobiol, 2024, 61(12): 10792-10804. DOI:10.1007/s12035-024-04225-1 |
| [74] | Pan JR, Peng JL, Li XP, et al. Transmission of NLRP3-IL-1β signals in cerebral ischemia and reperfusion injury: from microglia to adjacent neuron and endothelial cells via IL-1β/IL-1R1/TRAF6[J]. Mol Neurobiol, 2023, 60(5): 2749-2766. DOI:10.1007/s12035-023-03232-y |
| [75] | Wang L, Li RF, Guan XL, et al. The value of extracellular cold-inducible RNA-binding protein (eCIRP) in predicting the severity and prognosis of patients after cardiac arrest: a preliminary observational study[J]. Shock, 2021, 56(2): 229-236. DOI:10.1097/SHK.0000000000001702 |
| [76] | Tang XH, Ke J, Chen FL, et al. Hypoxic preconditioned mesenchymal stem cells ameliorate rat brain injury after cardiopulmonary resuscitation by suppressing neuronal pyroptosis[J]. J Cell Mol Med, 2023, 27(13): 1836-1858. DOI:10.1111/jcmm.17782 |
| [77] | Boissady E, Kohlhauer M, Lidouren F, et al. Ultrafast hypothermia selectively mitigates the early humoral response after cardiac arrest[J]. J Am Heart Assoc, 2020, 9(23): e017413. DOI:10.1161/JAHA.120.017413 |
| [78] | Chaban V, Nakstad ER, Stær-Jensen H, et al. Complement activation is associated with poor outcome after out-of-hospital cardiac arrest[J]. Resuscitation, 2021, 166: 129-136. DOI:10.1016/j.resuscitation.2021.05.038 |
| [79] | Wang L, Li RF, Guan XL, et al. Predictive value of soluble CD59 for poor 28-day neurological prognosis and all-cause mortality in patients after cardiopulmonary resuscitation: a prospective observatory study[J]. J Intensive Care, 2023, 11(1): 3. DOI:10.1186/s40560-023-00653-8 |
| [80] | de Kok MJC, Schaapherder AFM, Bloeme-Ter Horst JR, et al. Clinical ischemia-reperfusion injury: driven by reductive rather than oxidative stress? A narrative review[J]. Biochim Biophys Acta Bioenerg, 2025, 1866(2): 149539. DOI:10.1016/j.bbabio.2025.149539 |
| [81] | Xia PP, Marjan M, Liu ZY, et al. Chrysophanol postconditioning attenuated cerebral ischemia-reperfusion injury induced NLRP3-related pyroptosis in a TRAF6-dependent manner[J]. Exp Neurol, 2022, 357: 114197. DOI:10.1016/j.expneurol.2022.114197 |
| [82] | He YH, Chang Y, Peng YQ, et al. Glibenclamide directly prevents neuroinflammation by targeting SUR1-TRPM4-mediated NLRP3 inflammasome activation in microglia[J]. Mol Neurobiol, 2022, 59(10): 6590-6607. DOI:10.1007/s12035-022-02998-x |
| [83] | Xu JF, Zhang MH, Liu F, et al. Mesenchymal stem cells alleviate post-resuscitation cardiac and cerebral injuries by inhibiting cell pyroptosis and ferroptosis in a swine model of cardiac arrest[J]. Front Pharmacol, 2021, 12: 793829. DOI:10.3389/fphar.2021.793829 |
| [84] | Li JJ, Xu PF, Hong Y, et al. Lipocalin-2-mediated astrocyte pyroptosis promotes neuroinflammatory injury via NLRP3 inflammasome activation in cerebral ischemia/reperfusion injury[J]. J Neuroinflammation, 2023, 20(1): 148. DOI:10.1186/s12974-023-02819-5 |
| [85] | Li WY, Shen N, Kong LQ, et al. STING mediates microglial pyroptosis via interaction with NLRP3 in cerebral ischaemic stroke[J]. Stroke Vasc Neurol, 2024, 9(2): 153-164. DOI:10.1136/svn-2023-002320 |
| [86] | Liao YC, Hu JP, Guo C, et al. Acteoside alleviates blood-brain barrier damage induced by ischemic stroke through inhibiting microglia HMGB1/TLR4/NLRP3 signaling[J]. Biochem Pharmacol, 2024, 220: 115968. DOI:10.1016/j.bcp.2023.115968 |
| [87] | Zheng GH, Xu J, He FL, et al. Effects of NLRP3 inflammasome blockade on postresuscitation cerebral function in a rat model of cardiopulmonary resuscitation[J]. Biomed Pharmacother, 2021, 143: 112093. DOI:10.1016/j.biopha.2021.112093 |
| [88] | You GX, Zheng LB, Zhang YY, et al. Tangeretin attenuates cerebral ischemia-reperfusion-induced neuronal pyroptosis by inhibiting AIM2 inflammasome activation via regulating NRF2[J]. Inflammation, 2024, 47(1): 145-158. DOI:10.1007/s10753-023-01900-8 |
| [89] | Shao RJ, Wang XT, Xu TH, et al. The balance between AIM2-associated inflammation and autophagy: the role of CHMP2A in brain injury after cardiac arrest[J]. J Neuroinflammation, 2021, 18(1): 257. DOI:10.1186/s12974-021-02307-8 |
| [90] | Zhang H, Zhu C, Zhou XY, et al. Edaravone dexborneol protected neurological function by targeting NRF2/ARE and NF-κB/AIM2 pathways in cerebral ischemia/reperfusion injury[J]. Front Pharmacol, 2025, 16: 1581320. DOI:10.3389/fphar.2025.1581320 |
| [91] | Xu SY, Bian HJ, Shu S, et al. AIM2 deletion enhances blood-brain barrier integrity in experimental ischemic stroke[J]. CNS Neurosci Ther, 2021, 27(10): 1224-1237. DOI:10.1111/cns.13699 |
| [92] | Monard C, Bianchi N, Poli E, et al. Cytokine hemoadsorption with CytoSorb® in post-cardiac arrest syndrome, a pilot randomized controlled trial[J]. Crit Care, 2023, 27(1): 36. DOI:10.1186/s13054-023-04323-x |
| [93] | Obling LER, Beske RP, Meyer MAS, et al. Inflammatory response after prehospital high-dose glucocorticoid to patients resuscitated from out-of-hospital cardiac arrest: a sub-study of the STEROHCA trial[J]. Resuscitation, 2024, 202: 110340. DOI:10.1016/j.resuscitation.2024.110340 |
| [94] | Jiang MR, Li R, Lyu JJ, et al. MCC950, a selective NLPR3 inflammasome inhibitor, improves neurologic function and survival after cardiac arrest and resuscitation[J]. J Neuroinflammation, 2020, 17(1): 256. DOI:10.1186/s12974-020-01933-y |
| [95] | Xia YY, Zou CM, Kang WC, et al. Invasive metastatic tumor-camouflaged ROS responsive nanosystem for targeting therapeutic brain injury after cardiac arrest[J]. Biomaterials, 2024, 311: 122678. DOI:10.1016/j.biomaterials.2024.122678 |
| [96] | Wider JM, Gruley E, Morse PT, et al. Modulation of mitochondrial function with near-infrared light reduces brain injury in a translational model of cardiac arrest[J]. Crit Care, 2023, 27(1): 491. DOI:10.1186/s13054-023-04745-7 |
| [97] | Aoki T, Endo Y, Nakamura E, et al. Therapeutic potential of mitochondrial transplantation in modulating immune responses post-cardiac arrest: a narrative review[J]. J Transl Med, 2024, 22(1): 230. DOI:10.1186/s12967-024-05003-2 |
| [98] | Hayashida K, Takegawa R, Endo Y, et al. Exogenous mitochondrial transplantation improves survival and neurological outcomes after resuscitation from cardiac arrest[J]. BMC Med, 2023, 21(1): 56. DOI:10.1186/s12916-023-02759-0 |
| [99] | Wang ZR, Du J, Lachance BB, et al. Intracerebroventricular administration of hNSCs improves neurological recovery after cardiac arrest in rats[J]. Stem Cell Rev Rep, 2021, 17(3): 923-937. DOI:10.1007/s12015-020-10067-w |
| [100] | Wang ZR, Zhang S, Du J, et al. Neuroprotection of NSC therapy is superior to glibenclamide in cardiac arrest-induced brain injury via neuroinflammation regulation[J]. Transl Stroke Res, 2023, 14(5): 723-739. DOI:10.1007/s12975-022-01047-y |
 2025, Vol. 34
2025, Vol. 34