2. 浙江省人民医院(杭州医学院附属人民医院)急诊科,杭州 310015;
3. 浙江大学医学院附属第二医院急诊科 浙江省严重创伤与烧伤诊治重点实验室 浙江省急危重症临床医学研究中心,杭州 310009;
4. 纽约州立大学上州医科大学外科系,雪城 13210
2. Department of Emergency, Zhejiang Provincial People's Hospital, Affiliated People's Hospital, Hangzhou Medical College, Hangzhou 310015, China;
3. Department of Emergency Medicine, Second Affiliated Hospital, Zhejiang University School of Medicine, Key Laboratory of The Diagnosis and Treatment of Severe Trauma and Burn of Zhejiang Province, Zhejiang Province Clinical Research Center for Emergency and Critical Care Medicine, Hangzhou 310009, China;
4. Department of Surgery, State University of New York Upstate Medical University, Syracuse 13210, USA
脓毒症是指机体对感染的反应失调而导致危及生命的器官功能障碍[1],肺是脓毒症时最易受损伤的靶器官,脓毒症相关急性肺损伤(acute lung injury,ALI)发病率高,病死率高,也一直是临床治疗上的痛点和难点[2-3]。课题组前期研究发现肺表面活性蛋白D(surfactant associated protein D,SP-D)在ALI的发生发展中扮演了重要角色[4],能通过清除病原体和调节免疫反应,参与调控肺部的先天免疫防御[5],是ALI潜在的治疗靶标。
胰高血糖素样肽-1(glucagon-like peptide-1, GLP-1)是肠道L细胞分泌的一种肠肽激素。利拉鲁肽是一种GLP-1类似物,可结合并激活GLP-1受体,发挥类似GLP-1作用,目前在临床上主要用于促进胰岛素的分泌和合成,发挥调节血糖作用[6]。此外利拉鲁肽被发现还具体抗炎、抗凋亡、神经保护等多种药理作用[7-8],在ALI模型中,有研究发现利拉鲁肽可通过抑制TLR4信号通路对脂多糖诱导的小鼠ALI模型起保护作用[9],但关于利拉鲁肽在小鼠ALI模型中对SP-D表达和分泌的影响目前尚未见报道。本研究主要观察利拉鲁肽对脓毒症小鼠ALI的保护作用及其对SP-D表达和分泌的影响,以期为ALI的治疗提供新的思路。
1 材料与方法 1.1 主要材料 1.1.1 实验动物SPF级健康雄性FVB/NJ小鼠36只,8~12周龄,体重22~30 g,购自美国Jackson实验室,在美国SUNY Upstate Medical University动物中心屏障系统中饲养,环境温度22~24 ℃,相对湿度60%,12 h人工日光灯管照射,小鼠自由饮水和进食。
1.1.2 主要试剂和设备铜绿假单胞菌菌株由美国上州医科大学Jennifer教授馈赠,利拉鲁肽注射液购自丹麦诺和诺德制药有限公司,RIPA蛋白裂解液、BCA蛋白浓度测定试剂盒和ELISA试剂盒(TNF-α、IL-6)购自美国Thermo Scientific公司,兔抗鼠SP-D抗体由美国杜克大学Wright教授馈赠,β-actin一抗、辣根过氧化物酶标记山羊抗兔IgG的荧光二抗和Western blot二抗购自英国Abcam公司。主要设备包括倒置荧光显微镜购自日本Olympus公司,SDS-PAGE电泳仪和电转仪购自美国Bio-rad公司。
1.2 实验方法 1.2.1 模型制备及分组将适应性喂养1周后的小鼠采用随机数字法分为3组,正常对照组(Control组)、急性肺损伤组(Acute lung injury group,ALI组)和利拉鲁肽干预组(ALI+LIRA组),每组12只;根据课题组既往研究方法制备脓毒症致急性肺损伤小鼠模型[10-11],即先将小鼠腹腔注射戊巴比妥钠麻醉,ALI组和ALI+LIRA组小鼠给予气管内注射铜绿假单胞菌混悬液50 µL(5×104 CFU/mice),Control组给予气管内滴注等体积生理盐水;ALI+LIRA组在造模后0.5 h给予小鼠利拉鲁肽(2 mg/kg)皮下注射干预,Control组和ALI组给予等体积生理盐水皮下注射。造模后小鼠出现呼吸急促、精神萎靡和进食进水减少等典型脓毒症样临床表现。3组小鼠均于术后24 h处死并取材。本实验已经通过SUNY Upstate Medical University伦理委员会批准,批准号是IACUC#380。
1.2.2 肺组织HE染色提取小鼠左肺组织,4%多聚甲醛固定,逐级乙醇脱水,二甲苯透明、浸蜡,石蜡包埋,制成4 mm的石蜡切片,使用HE进行染色,光镜下观察肺组织形态学改变。
1.2.3 BALF提取及总蛋白浓度测定造模后24 h对小鼠进行支气管肺泡灌洗,连续3次,利用BCA法测定BALF中总蛋白浓度,250 g离心10 min后取上清液,-80 ℃冰箱存储。
1.2.4 酶联免疫吸附法测定TNF-α、IL-6水平取冻存的BALF标本,解冻后将相关样品、标准品、质控品和稀释缓冲液等加入酶反应孔,孵育抗体,充分洗涤,加入底物液显色,于波长450 nm处读取吸光度,绘制标准曲线,计算出样品实际浓度。
1.2.5 免疫荧光法测定SP-D免疫荧光定位取制备好的肺组织石蜡切片,抗原修复后降至室温,体积分数10%的驴血清室温孵育1 h后滴加SP-D一抗(1:200),4 ℃孵育过夜,次日复温1 h后,再加入Alexa 488标记的二抗(1:500),避光孵育1 h,PBS洗涤3次,抗荧光淬灭封片剂封片,倒置荧光显微镜下拍照观察。
1.2.6 Western blot测定SP-D表达取-80 ℃冻存的肺组织和BALF,加入RIPA裂解液充分裂解后,匀浆,4 ℃13 000 g离心10 min,取上清液用BCA法测蛋白浓度,制备样品;样品上样后经SDS-PAGE凝胶电泳分离蛋白质,并转至PVDF膜上,5%脱脂奶粉室温下封闭1 h后,加入兔抗鼠SP-D及β-actin一抗4 ℃孵育过夜,TBST洗膜三次,加入HRP标记的羊抗兔二抗孵育1 h,TBST洗膜3次,暗室内ECL化学发光法显色、曝光并扫描,采用Image J软件分析蛋白条带灰度值并定量分析。
1.3 统计学方法使用SPSS22.0统计软件(IBM公司,美国)进行数据统计分析。符合正态分布的资料以均数±标准差(x±s)表示,组间比较采用独立样本t检验,非正态分布资料以中位数表示,组间比较采用非参数秩和检验。以P < 0.05为差异有统计学意义。
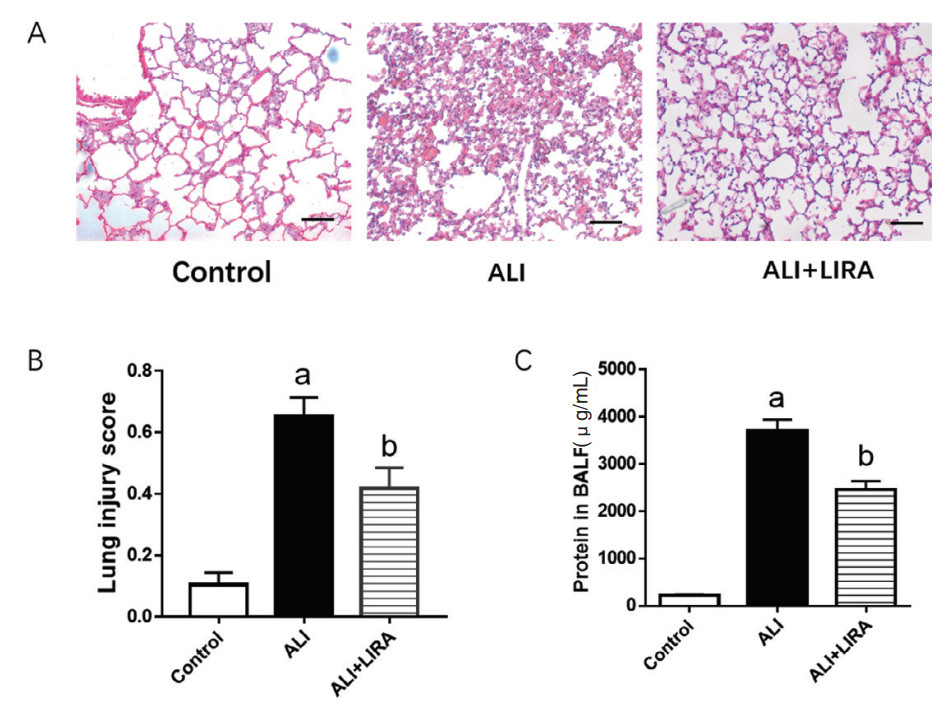
2 结果 2.1 各组小鼠肺脏组织病理学改变及BALF中总蛋白浓度变化光镜下,Control组小鼠肺泡腔完好,肺泡壁结构完整,肺泡腔内无分泌物;与对照组相比,ALI组小鼠可见肺组织结构严重破坏、肺间质充血水肿、肺泡腔变窄和肺泡腔内大量的炎性细胞浸润;与ALI组相比,ALI+LIRA组肺组织损伤减轻,肺间质水肿减轻,肺泡腔内炎性细胞减少,差异均有统计学意义(P < 0.05), 见图 1A。BCA法测定蛋白浓度结果显示,ALI组小鼠BALF中总蛋白浓度较Control组升高明显,利拉鲁肽干预后,ALI+LIRA组总蛋白浓度较ALI组降低,差异均有统计学意义(P < 0.05),见图 1C。
|
| A: 肺组织病理改变(HE×200);B: 肺组织损伤评分;C:BALF中总蛋白浓度;与Control组比较,aP < 0.05; 与ALI组比较,bP < 0.05 图 1 各组小鼠肺组织病理学和BALF中总蛋白浓度比较 Fig 1 Comparison of lung tissue pathology and total protein concentration in BALF in each group |
|
|
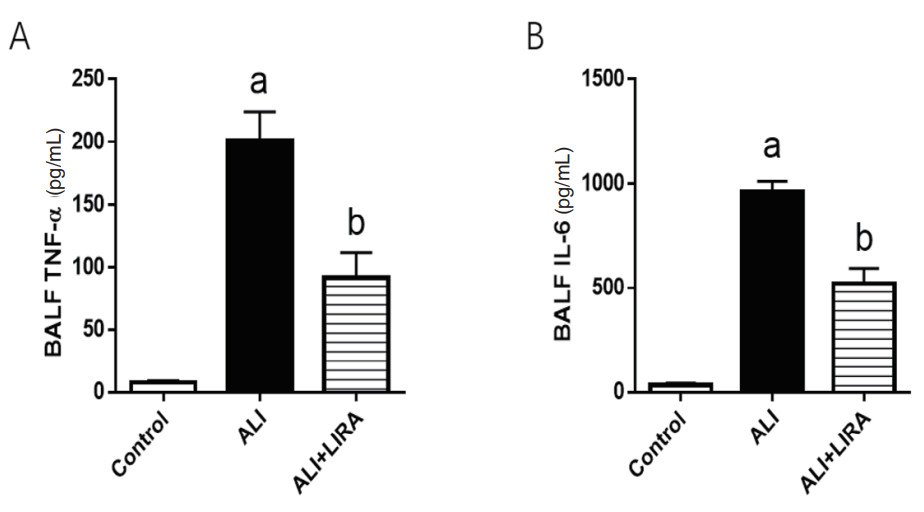
ELISA法检测BALF中TNF-α和IL-6炎症因子的含量。与Control组比较,ALI组小鼠BALF中TNF-α和IL-6升高明显;与ALI组比较,利拉鲁肽干预后,ALI+LIRA组TNF-α和IL-6降低明显,差异均有统计学意义(P < 0.05),见图 2。
|
| A: TNF-α为肿瘤坏死因子α;B:IL-6为白细胞介素6;与Control组比较,aP < 0.05; 与ALI组比较,bP < 0.05 图 2 各组小鼠BALF中炎症因子比较 Fig 2 Comparison of inflammatory factors in BALF in each group |
|
|
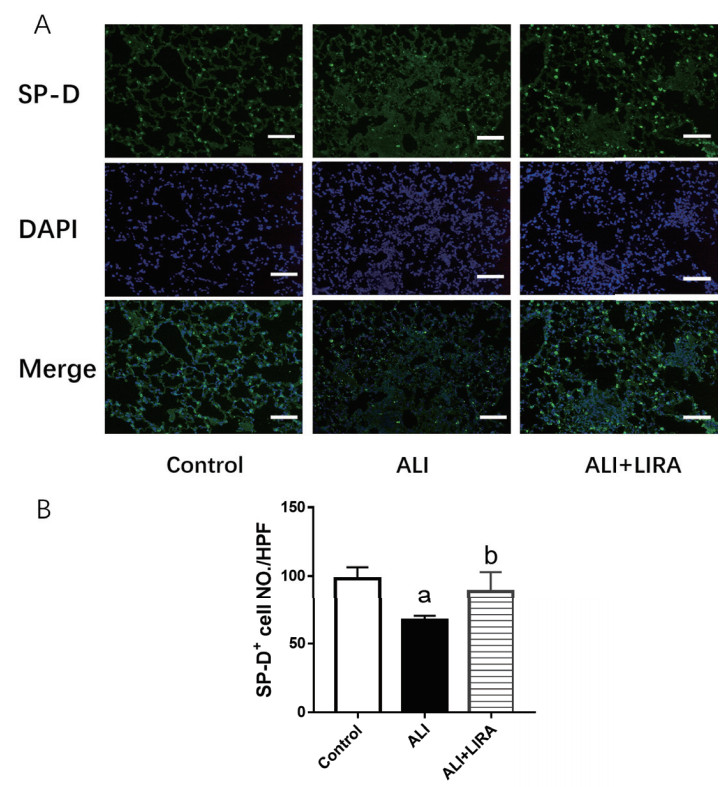
采用免疫荧光定位法检测各组小鼠肺组织SP-D定位表达情况,结果显示:与Control组比较,ALI组小鼠肺组织中SP-D表达阳性细胞减少明显;与ALI组比较,利拉鲁肽干预后,ALI+LIRA组SP-D表达阳性细胞增多明显,差异均有统计学意义(P < 0.05),见图 3。
|
| A: 各组小鼠肺组织SP-D免疫荧光染色结果(×200);B: 各组小鼠SP-D阳性细胞数比较;与Control组比较,aP < 0.05; 与ALI组比较,bP < 0.05 图 3 各组小鼠肺组织SP-D免疫荧光定位比较 Fig 3 Comparison of SP-D immunofluorescence in the lung in each group |
|
|
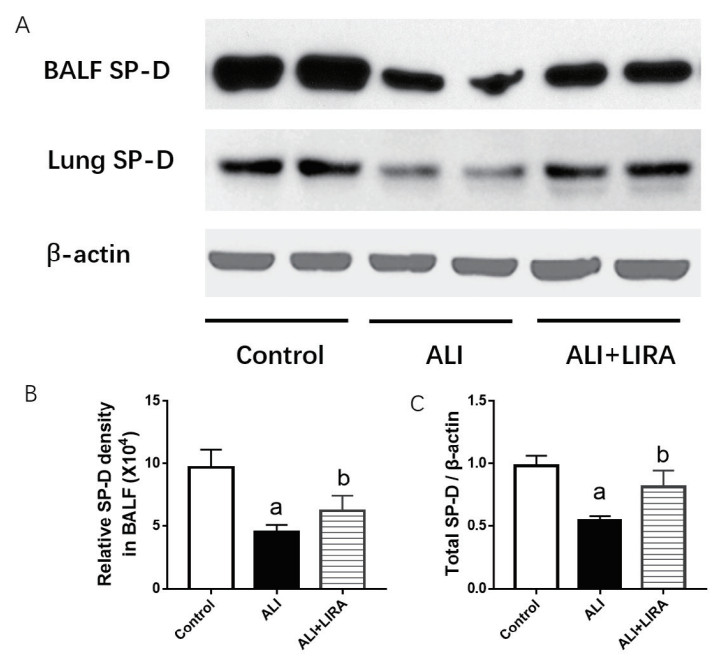
采用免疫蛋白印迹法检测各组小鼠BALF和肺组织中SP-D在蛋白水平的表达变化,结果显示:与Control组比较,ALI组小鼠BALF中分泌的SP-D和肺组织中SP-D蛋白水平均减少明显;与ALI组比较,利拉鲁肽干预后,ALI+LIRA组BALF中分泌的SP-D和肺组织中SP-D蛋白水平回升明显,差异均有统计学意义(P < 0.05),见图 4。
|
| A: 各组小鼠BALF和肺组织中SP-D表达;B: BALF中SP-D表达定量分析;C: 肺组织中SP-D表达定量分析;与Control组比较,aP < 0.05; 与ALI组比较,bP < 0.05 图 4 各组小鼠BALF和肺组织SP-D表达变化 Fig 4 Comparison of SP-D expression in BALF and the lung in each group |
|
|
利拉鲁肽作为GLP-1受体激动剂,目前在临床上已经广泛用于糖尿病等代谢性相关疾病的治疗。由于GLP-1受体在人体内广泛表达,除了胰腺有丰富表达之外,GLP-1受体在肺脏、大脑和肾脏等脏器也显著表达,因此GLP-1受体激动剂还具有广泛药理作用[12],在脓毒症持续性炎症反应-免疫抑制-分解代谢综合征中也发挥了重要作用[13]。Viby等[14]的研究证实GLP-1受体激动剂能通过改善COPD模型小鼠的肺功能从而减少其病死率;而Baer等[15]的研究表明,GLP-1受体激动剂预处理改善了脓毒症小鼠的肺部炎症,减少了肺泡毛细血管屏障破坏和肺损伤;而Feng等[16]研究发现利拉鲁肽联合间充质干细胞能通过cAMP/PKAc/β-catenin信号通路减轻小鼠ALI肺部损伤。本研究结果则证实了利拉鲁肽干预后能改善脓毒症相关ALI小鼠肺组织病理学改变,肺组织损伤评分明显下降,与既往研究结果相一致[17]。
肺表面活性物质(pulmonary surfactant,PS)是由肺泡Ⅱ型上皮细胞分泌的一种磷脂蛋白混合物,其主要成分是二棕榈酰卵磷脂(占90%)和表面活性物质相关蛋白(占10%),在维持肺泡稳定性、降低肺泡张力和参与调节肺组织宿主防御等过程中发挥重要作用[18]。在2001年Vara等[19]就发现GLP-1(7-36)代谢产物能刺激人的肺泡Ⅱ型上皮细胞PS的分泌,但由于GLP-1在体内很容易降解以及人肺泡Ⅱ型细胞难以分离,因此后续再无类似相关研究。直到利拉鲁肽上市后,Romaní-Pérez等[20]研究发现,在Ⅰ型糖尿病小鼠给予利拉鲁肽干预后能增加其肺部SP-A和SP-B蛋白的表达;而Fandino等[21]研究表明,对于食物限制模型的母鼠模型中,应用利拉鲁肽能够减少SP-A的丢失从而改善子代的存活率;Zhu等[22]研究发现,在LPS诱导小鼠ALI模型中,利拉鲁肽能通过TTF-1信号通路增加SP-A的表达而减轻小鼠肺部炎症水平。本研究则发现,经过利拉鲁肽干预后,ALI+LIRA组小鼠肺部表达SP-D阳性的肺泡Ⅱ型细胞数量明显增多,并且TNF-α和IL-6炎症因子水平均较ALI组下降,提示利拉鲁肽对SP-D的表达也有促进作用。
本研究通过直接向小鼠气管内注射铜绿假单胞菌构建脓毒症ALI模型,与传统腹腔内注射LPS或盲肠结扎穿孔术构建ALI模型相比,本模型与临床致病过程更接近[23],也更符合ALI病理生理过程。而在本模型中利拉鲁肽干预后,发现ALI小鼠肺组织内的肺泡Ⅱ型上皮细胞内合成的SP-D水平提高,同时分泌到BALF中真正发挥宿主免疫作用的SP-D水平也明显提升,这提示利拉鲁肽在ALI中对SP-D的表达和分泌都可能有促进作用。SP-D是肺表面活性物质相关蛋白中主要参与宿主防御的主要活性蛋白,是一种含有胶原的C型凝集素。SP-D可通过直接与病原体或相关细胞受体结合,调节宿主免疫细胞反应,增强巨噬细胞的吞噬功能,发挥抗菌作用并抑制炎症水平[24]。脓毒症时,由于炎性因子直接或间接损伤肺泡Ⅱ型上皮细胞,干扰SP-D等PS的合成和分泌,SP-D水平降低会进一步加重肺损伤,因此体循环中SP-D水平也作为ALI的生物标志物[25],另一方面外源性重组SP-D也是治疗ALI的潜在靶点[26]。本研究则证实利拉鲁肽在脓毒症中能促进SP-D的表达和分泌,但这些作用是否和肺泡巨噬细胞的自噬、吞噬活化等功能是否相关,目前尚不清楚,具体机制仍有待我们进一步去研究证实。此外本研究还存在研究样本量偏少和研究周期偏短等不足,其长期效果和潜在风险仍不清楚,有待进一步研究阐明。
利益冲突 所有作者声明无利益冲突
作者贡献声明 郭君平:研究设计、实施研究、论文撰写;潘然、王丽君、郑悦亮:数据整理分析、图表制作、论文撰写;张茂、王桂荣:研究设计、论文修改、经费支持
| [1] | Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3)[J]. JAMA, 2016, 315(8): 801-810. DOI:10.1001/jama.2016.0287 |
| [2] | Xie JF, Wang HL, Kang Y, et al. The epidemiology of sepsis in Chinese ICUs: a national cross-sectional survey[J]. Crit Care Med, 2020, 48(3): e209-e218. DOI:10.1097/CCM.0000000000004155 |
| [3] | 张建国, 陶志敏. 脓毒症的临床治疗进展—历经新冠疫情的思考[J]. 中华急诊医学杂志, 2024, 33(2): 135-144. DOI:10.3760/cma.j.issn.1671-0282.2024.02.002 |
| [4] | Yu J, Ni L, Zhang XY, et al. Surfactant protein D dampens lung injury by suppressing NLRP3 inflammasome activation and NF-κB signaling in acute pancreatitis[J]. Shock, 2019, 51(5): 557-568. DOI:10.1097/SHK.0000000000001244 |
| [5] | Du J, Abdel-Razek O, Shi Q, et al. Surfactant protein D attenuates acute lung and kidney injuries in pneumonia-induced sepsis through modulating apoptosis, inflammation and NF-κB signaling[J]. Sci Rep, 2018, 8(1): 15393. DOI:10.1038/s41598-018-33828-7 |
| [6] | Kelly AS, Auerbach P, Barrientos-Perez M, et al. A randomized, controlled trial of liraglutide for adolescents with obesity[J]. N Engl J Med, 2020, 382(22): 2117-2128. DOI:10.1056/NEJMoa1916038 |
| [7] | Pang J, Feng JN, Ling WH, et al. The anti-inflammatory feature of glucagon-like peptide-1 and its based diabetes drugs-Therapeutic potential exploration in lung injury[J]. Acta Pharm Sin B, 2022, 12(11): 4040-4055. DOI:10.1016/j.apsb.2022.06.003 |
| [8] | Song S, Guo RY, Mehmood A, et al. Liraglutide attenuate central nervous inflammation and demyelination through AMPK and pyroptosis-related NLRP3 pathway[J]. CNS Neurosci Ther, 2022, 28(3): 422-434. DOI:10.1111/cns.13791 |
| [9] | 喻双雪, 王文军, 孔玲玲, 等. 利拉鲁肽对细菌感染性脓毒症小鼠肺损伤的作用[J]. 中华医院感染学杂志, 2022, 32(20): 3041-3046. DOI:10.11816/cn.ni.2022-211851 |
| [10] | Yang FY, Zhang J, Yang Y, et al. Regulatory roles of human surfactant protein B variants on genetic susceptibility to Pseudomonas aeruginosa pneumonia-induced sepsis[J]. Shock, 2020, 54(4): 507-519. DOI:10.1097/SHK.0000000000001494 |
| [11] | Chen XH, Guo JP, Mahmoud S, et al. Regulatory roles of SP-A and exosomes in pneumonia-induced acute lung and kidney injuries[J]. Front Immunol, 2023, 14: 1188023. DOI:10.3389/fimmu.2023.1188023 |
| [12] | Zhao X, Wang MH, Wen ZT, et al. GLP-1 receptor agonists: beyond their pancreatic effects[J]. Front Endocrinol, 2021, 12: 721135. DOI:10.3389/fendo.2021.721135 |
| [13] | 刘红升, 张庆红. GLP-1在脓毒症持续性炎症反应-免疫抑制-分解代谢综合征中的作用机制[J]. 中华急诊医学杂志, 2023, 32(12): 1737-1741. DOI:10.3760/cma.j.issn.1671-0282.2023.12.030 |
| [14] | Viby NE, Isidor MS, Buggeskov KB, et al. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice[J]. Endocrinology, 2013, 154(12): 4503-4511. DOI:10.1210/en.2013-1666 |
| [15] | Baer B, Putz ND, Riedmann K, et al. Liraglutide pretreatment attenuates sepsis-induced acute lung injury[J]. Am J Physiol Lung Cell Mol Physiol, 2023, 325(3): L368-L384. DOI:10.1152/ajplung.00041.2023 |
| [16] | Feng Y, Wang LL, Ma XY, et al. Effect of hCMSCs and liraglutide combination in ALI through cAMP/PKAc/β-catenin signaling pathway[J]. Stem Cell Res Ther, 2020, 11(1): 2. DOI:10.1186/s13287-019-1492-6 |
| [17] | Zhou WY, Shao WJ, Zhang Y, et al. Glucagon-like peptide-1 receptor mediates the beneficial effect of liraglutide in an acute lung injury mouse model involving the thioredoxin-interacting protein[J]. Am J Physiol Endocrinol Metab, 2020, 319(3): E568-E578. DOI:10.1152/ajpendo.00292.2020 |
| [18] | Pioselli B, Salomone F, Mazzola G, et al. Pulmonary surfactant: a unique biomaterial with life-saving therapeutic applications[J]. Curr Med Chem, 2022, 29(3): 526-590. DOI:10.2174/0929867328666210825110421 |
| [19] | Vara E, Arias-Díaz J, Garcia C, et al. Glucagon-like peptide-1(7-36) amide stimulates surfactant secretion in human type Ⅱ pneumocytes[J]. Am J Respir Crit Care Med, 2001, 163(4): 840-846. DOI:10.1164/ajrccm.163.4.9912132 |
| [20] | Romaní-Pérez M, Outeiriño-Iglesias V, Moya CM, et al. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats[J]. Endocrinology, 2015, 156(10): 3559-3569. DOI:10.1210/en.2014-1685 |
| [21] | Fandiño J, Vaz AA, Toba L, et al. Liraglutide enhances the activity of the ACE-2/ang(1-7)/mas receptor pathway in lungs of male pups from food-restricted mothers and prevents the reduction of SP-A[J]. Int J Endocrinol, 2018, 2018: 6920620. DOI:10.1155/2018/6920620 |
| [22] | Zhu T, Li CY, Zhang X, et al. GLP-1 analogue liraglutide enhances SP-a expression in LPS-induced acute lung injury through the TTF-1 signaling pathway[J]. Mediators Inflamm, 2018, 2018: 3601454. DOI:10.1155/2018/3601454 |
| [23] | Qin MB, Gao YX, Guo SG, et al. Establishment and evaluation of animal models of sepsis-associated encephalopathy[J]. World J Emerg Med, 2023, 14(5): 349-353. DOI:10.5847/wjem.j.1920-8642.2023.088 |
| [24] | Sorensen GL. Surfactant protein D in respiratory and non-respiratory diseases[J]. Front Med, 2018, 5: 18. DOI:10.3389/fmed.2018.00018 |
| [25] | Elmore A, Almuntashiri A, Wang XY, et al. Circulating surfactant protein D: a biomarker for acute lung injury?[J]. Biomedicines, 2023, 11(9): 2517. DOI:10.3390/biomedicines11092517 |
| [26] | Beirag N, Kumar C, Madan T, et al. Human surfactant protein D facilitates SARS-CoV-2 pseudotype binding and entry in DC-SIGN expressing cells, and downregulates spike protein induced inflammation[J]. Front Immunol, 2022, 13: 960733. DOI:10.3389/fimmu.2022.960733 |
 2024, Vol. 33
2024, Vol. 33




